

Kidney Week 2012

Tolvaptan for ADPKD
Tolvaptan for ADPKD
“The High Impact Clinical Trials Session at this year’s American Society of Nephrology Renal Week was the site of one of the most important and exciting nephrology developments to materialize in years.
While the primary invstigator V. E Torrres was quick to temper enthusiasm until regulatory bodies weigh in, this study is a major breakthrough in the management of ADPKD and represents a shining example of bench to bedside research that will provide hope for a large group of patients who previously had no medical option for delaying the onset of end-stage renal disease in ADPKD.”


Here is what I wrote for eAJKD on the presentation
Kidney Week 2012: Late Breaking Trials: TEMPO
November 3, 2012 By
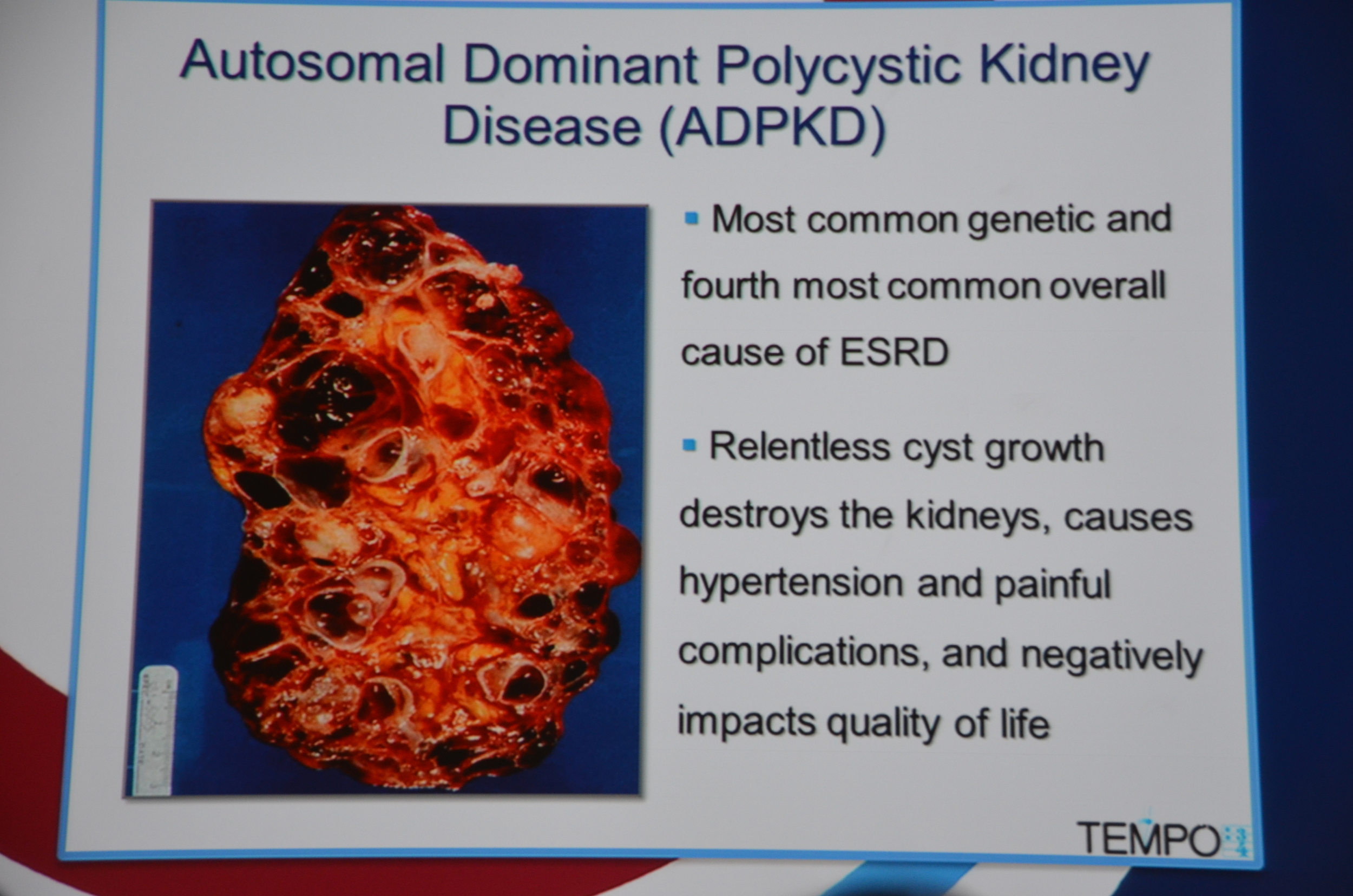
We now have an effective medication for autosomal dominant polycystic kidney disease. Tolvaptan, the oral vaptan approved for hyponatremia, was effective at slowing cyst growth and loss of GFR in the TEMPO 3:4 trial.
TEMPO stands for Tolvaptan Efficacy and safety in Management of Polycystic kidney disease and itsOutcomes.
The data was presented by the lead author, Vicente E. Torres of the Mayo clinic.
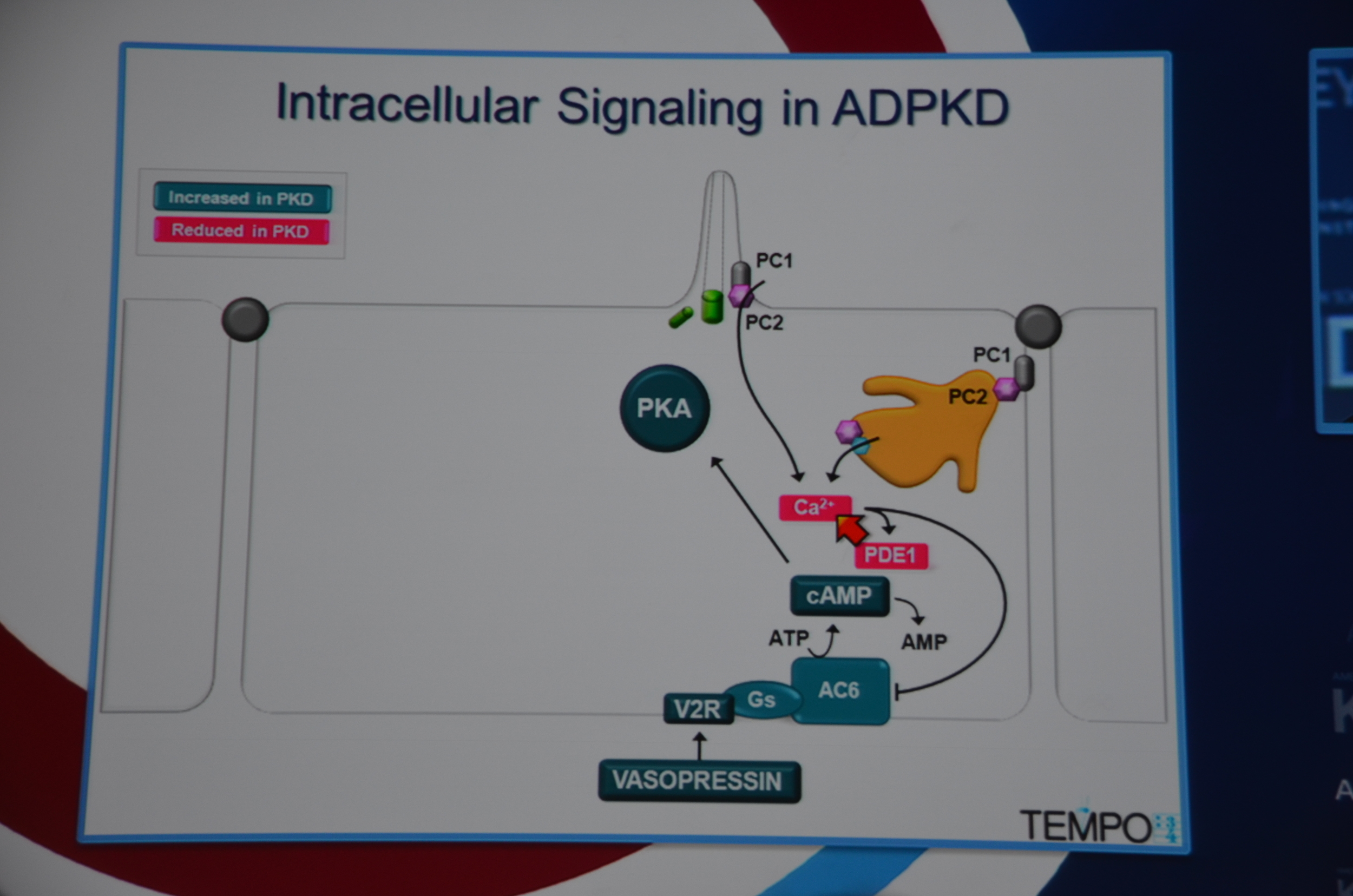
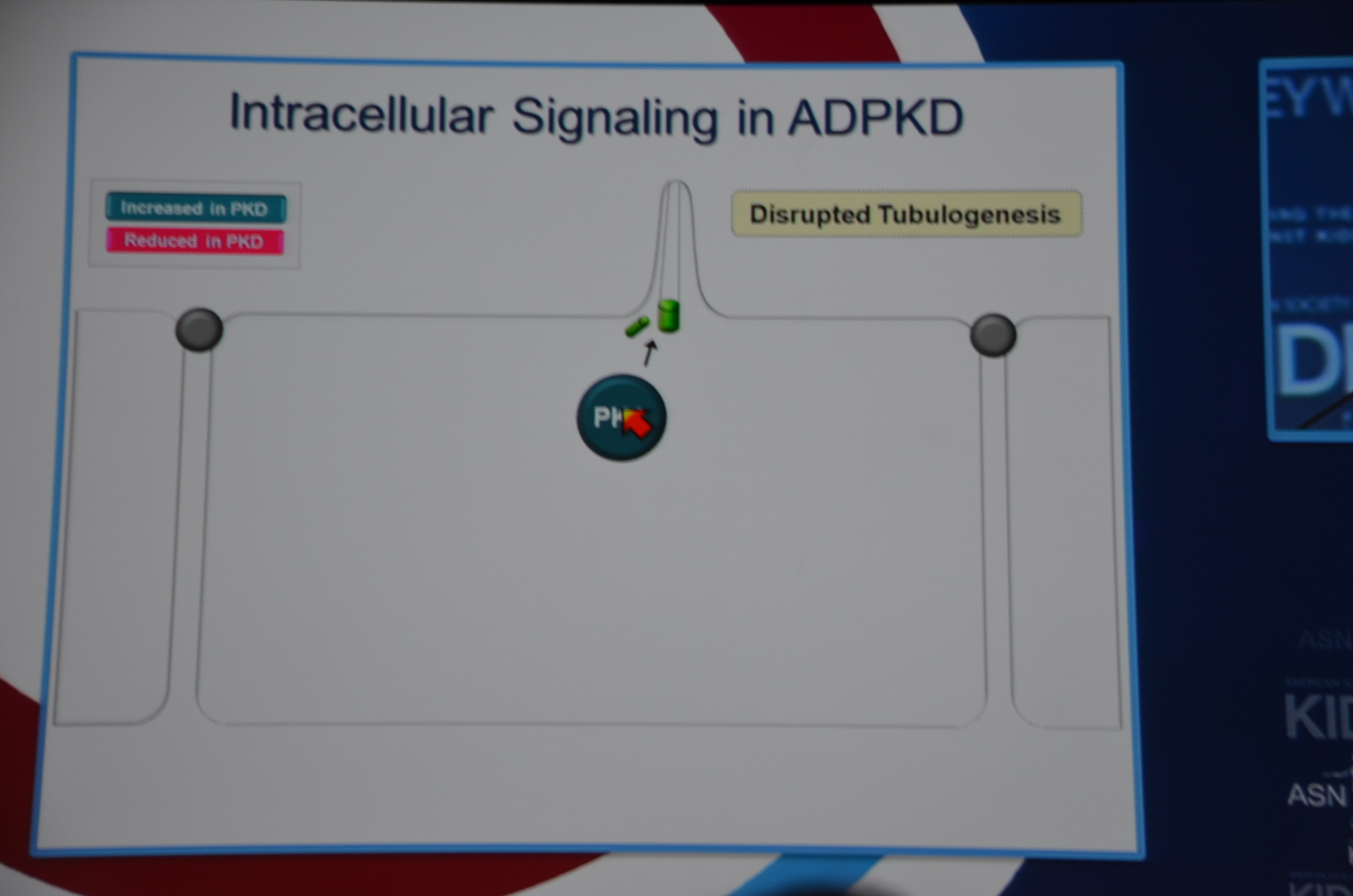
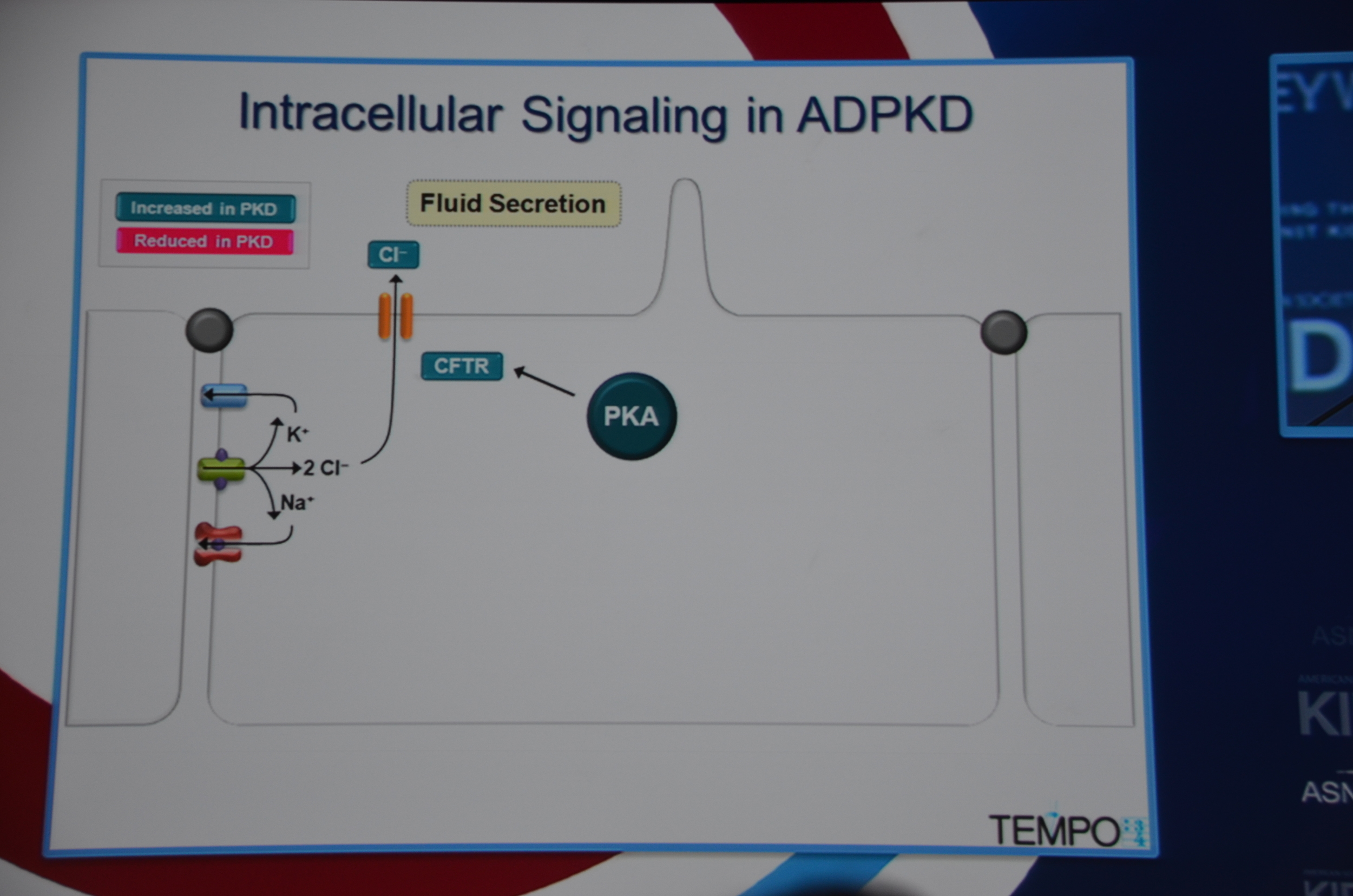
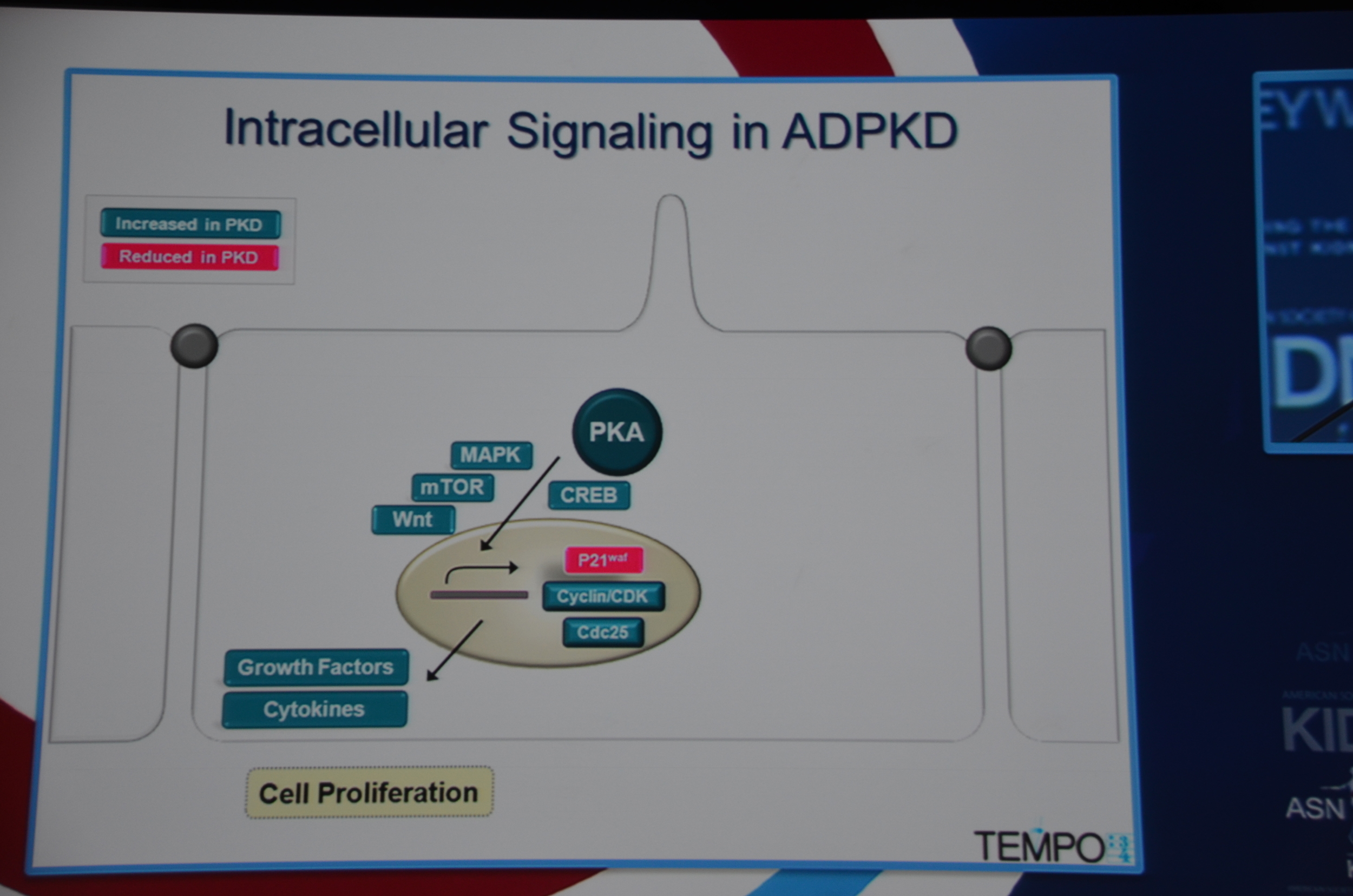
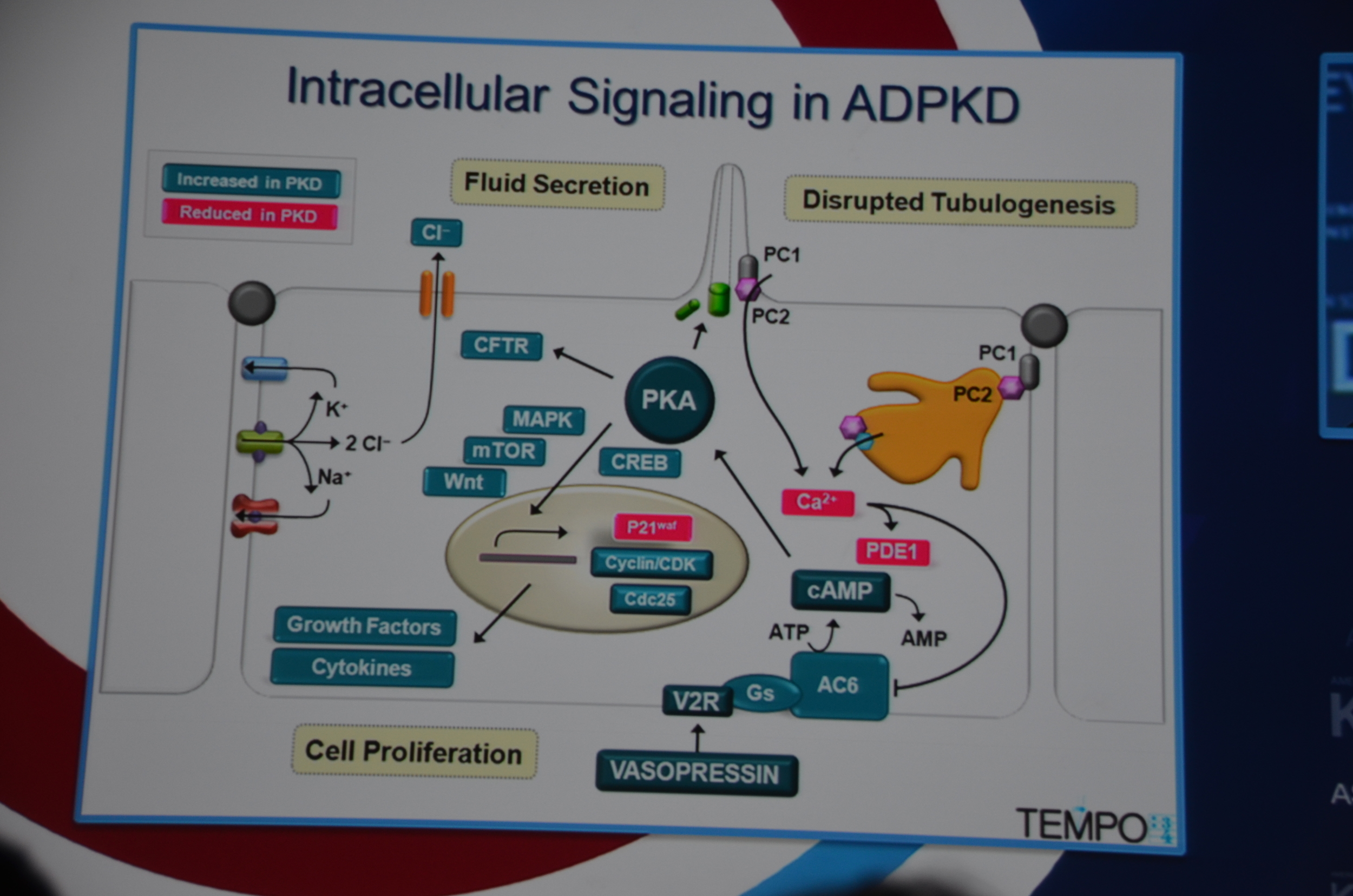
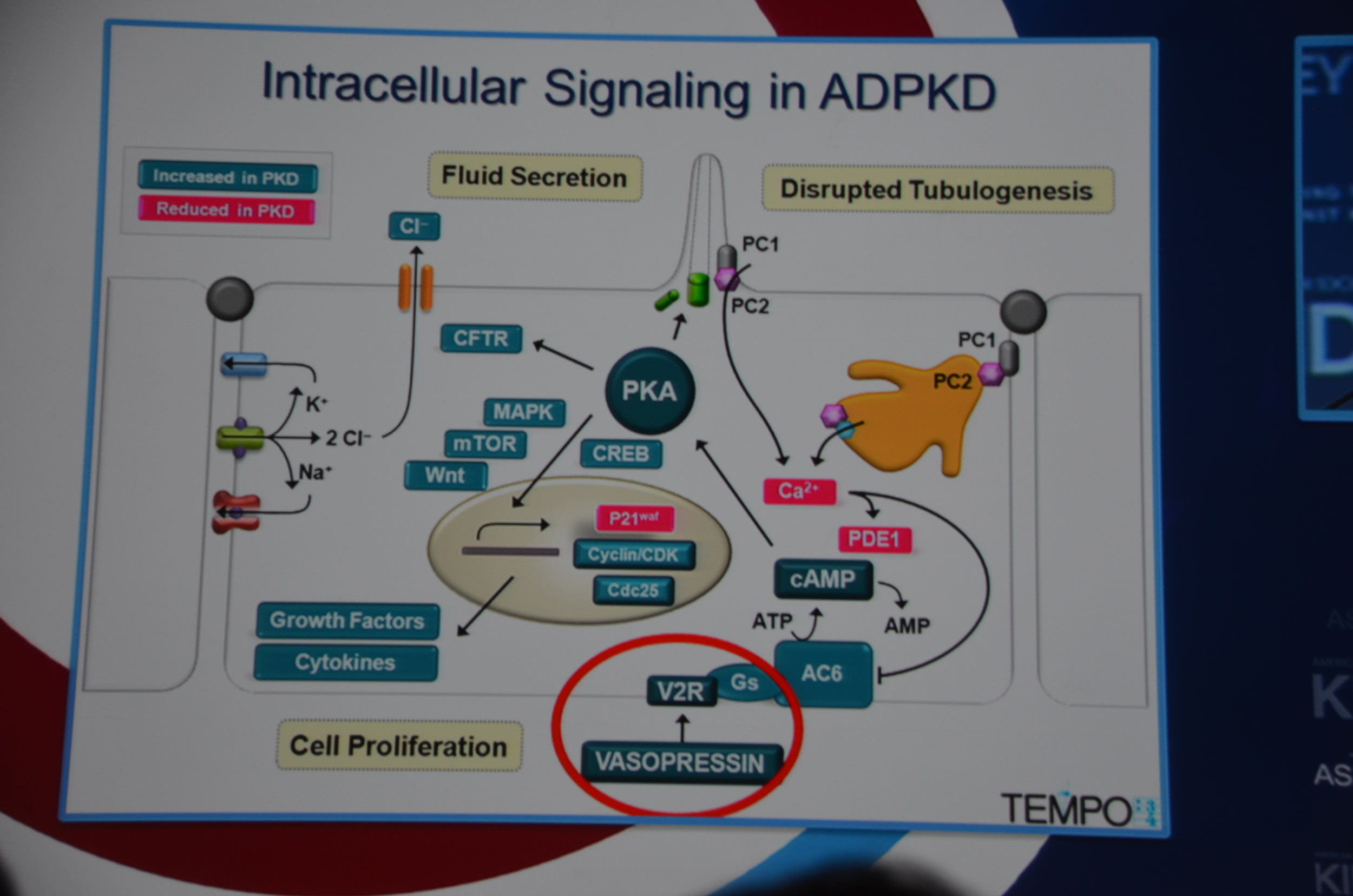
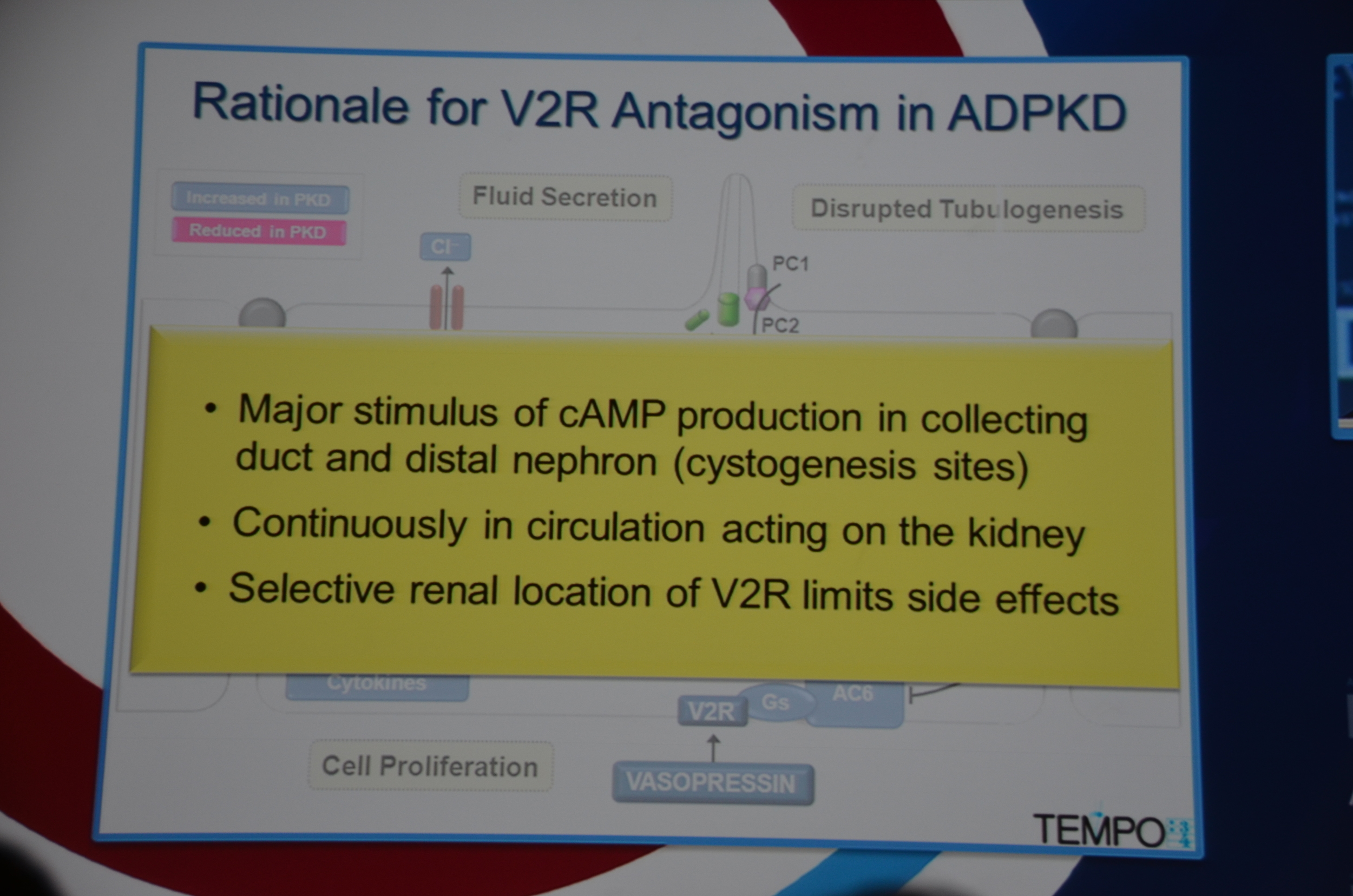
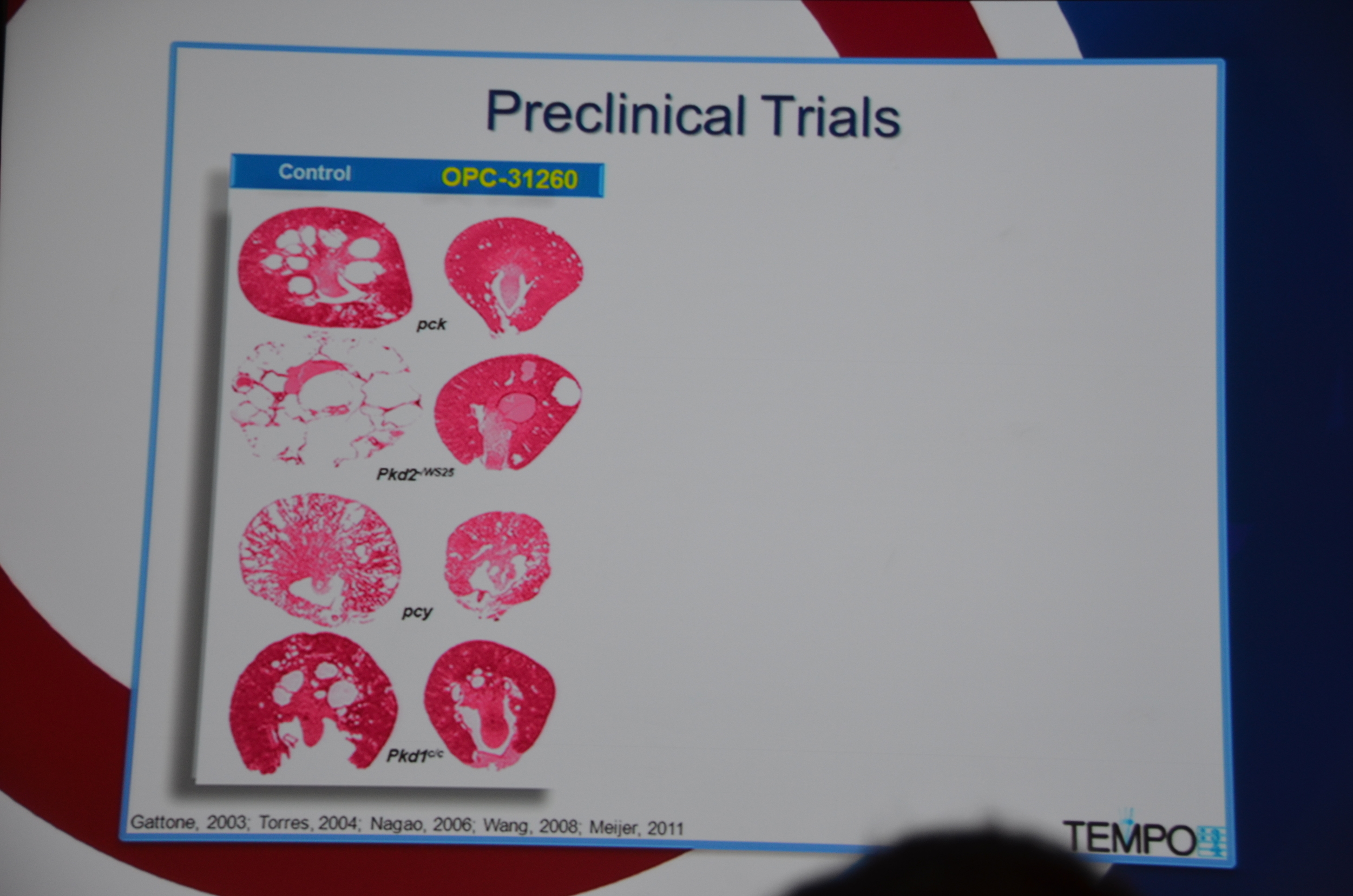
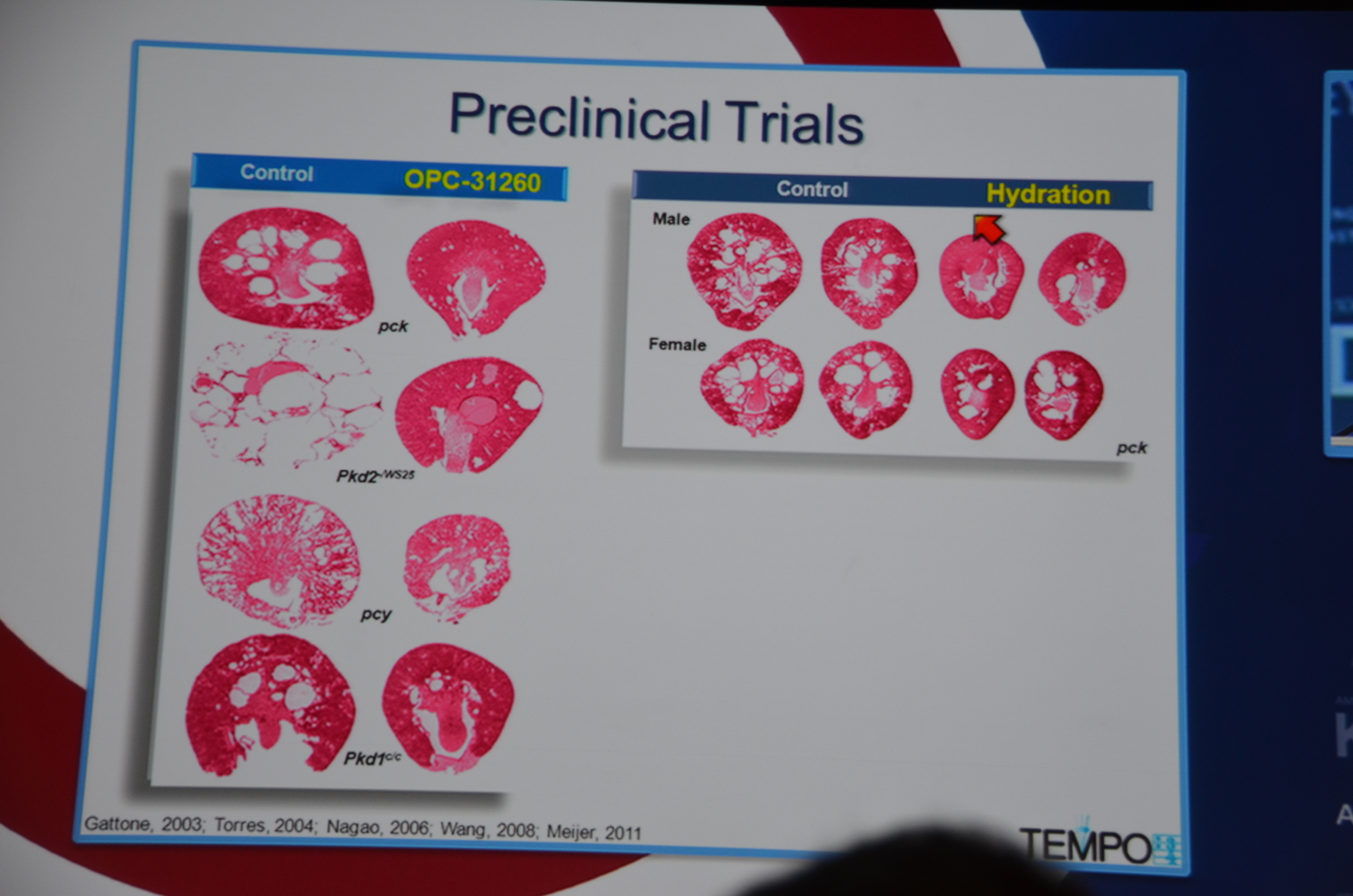
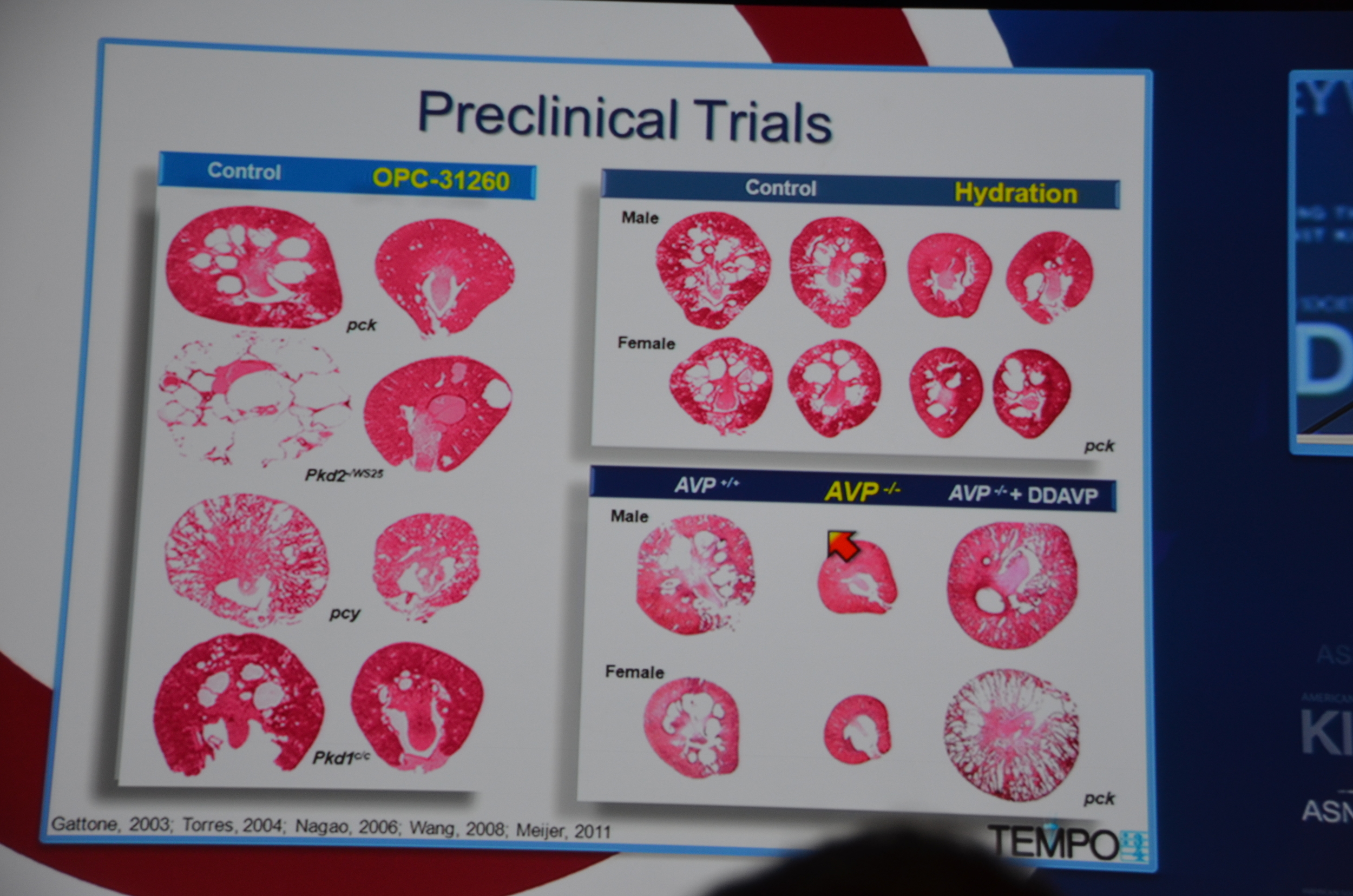
Torres begins by showing how vasopressin has a important role in stimulating cyclic AMP which via PKA causes cytogenesis by multiple mechanisms (disrupted tubulogenesis, increased fluid secretion, and stimulating cell proliferation). So the V2R is located at an important place in the pathogenesis of ADPKD but also, since it is limited to selective sites in the kidney, there are relatively few side effects.
Preclinical trials show that vasopressin antagonists decrease cyst growth in animals. Additionally, other mechanisms for decreasing vasopressin, such as increased water intake and knock out mice likewise decrease cyst growth. Data such as this led to the design of the TEMPO trial.
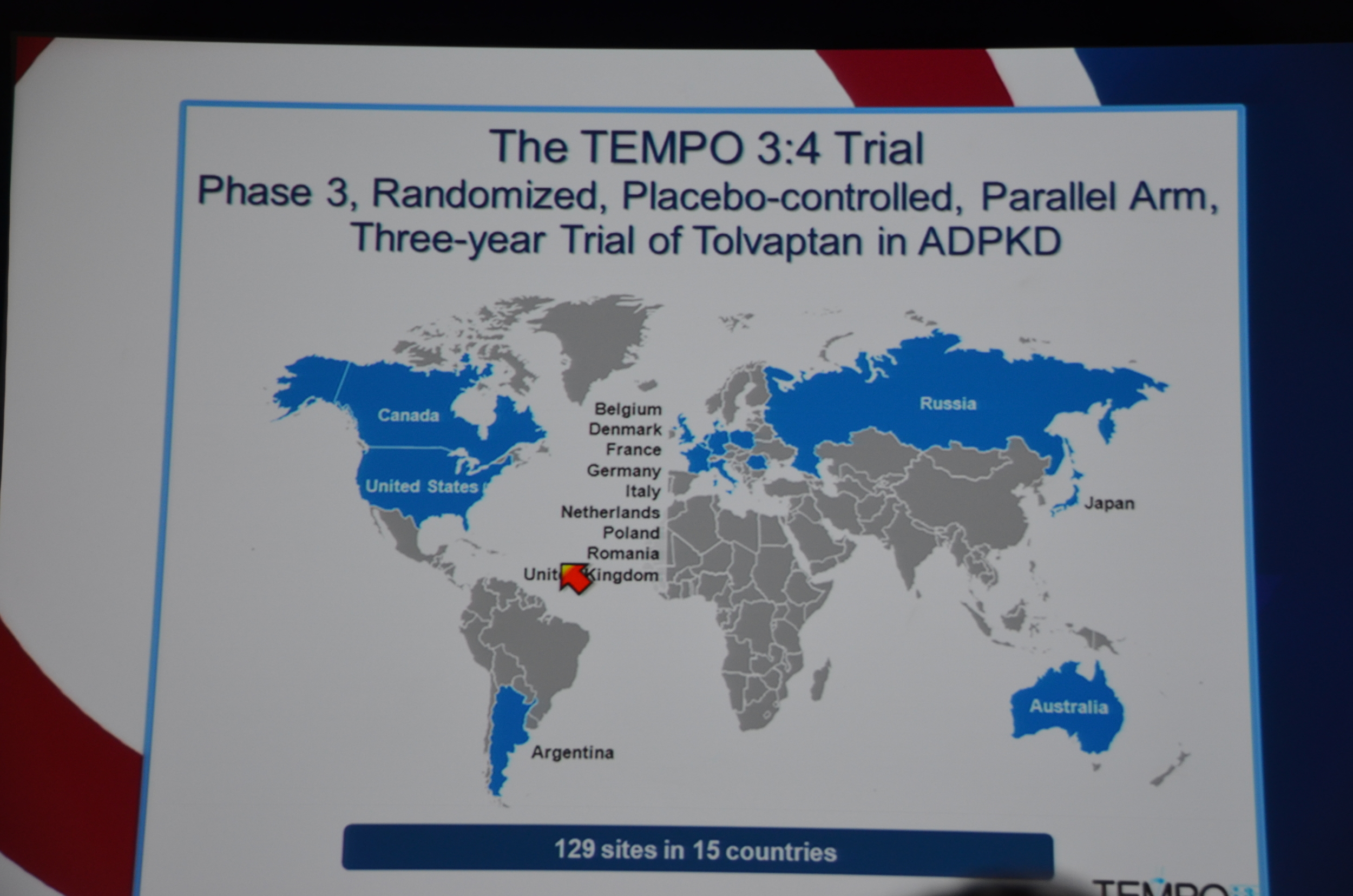
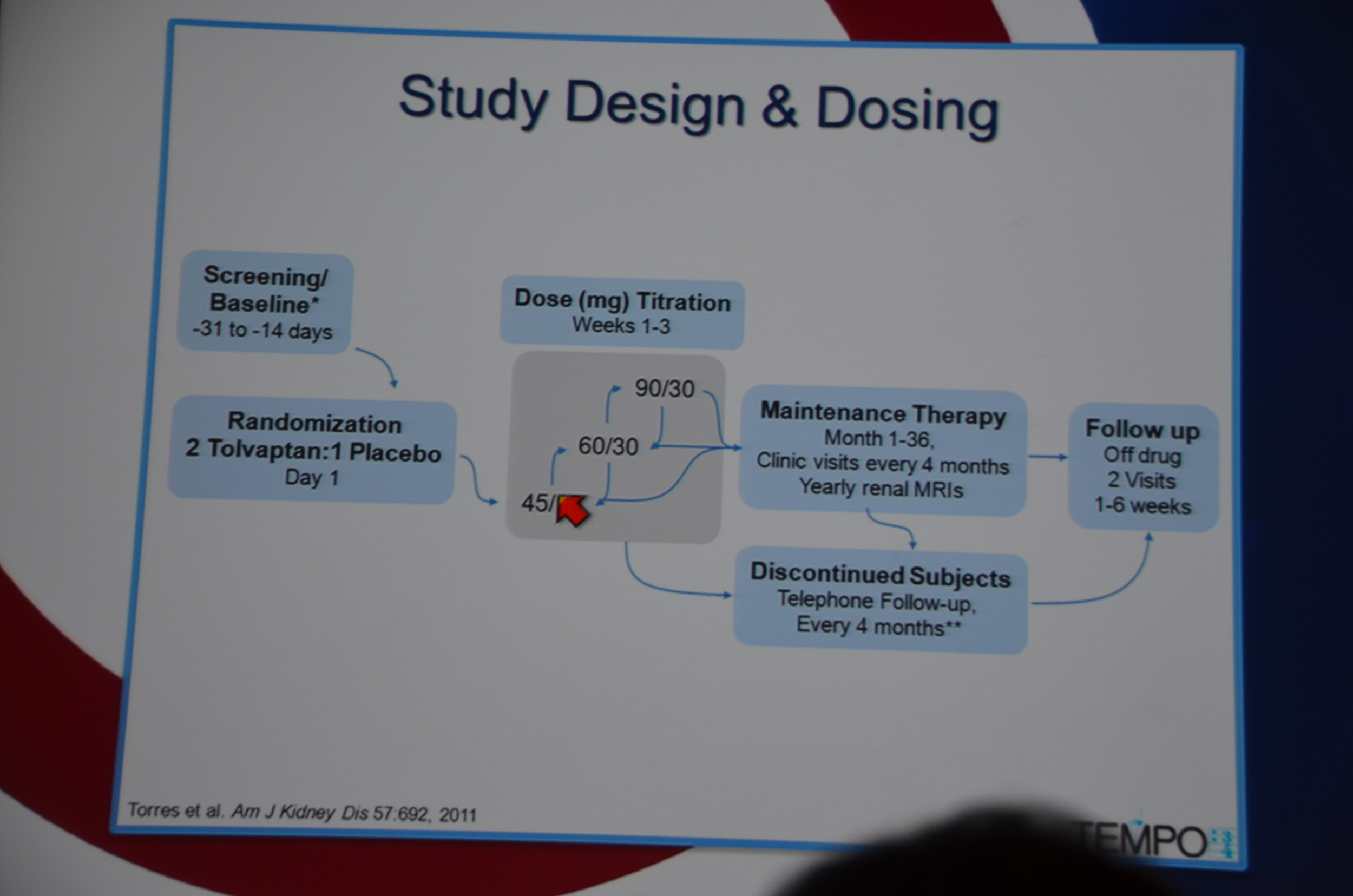
TEMPO 3:4 trial was a randomized, multinational, placebo controlled, parallel arm, three year trial.
129 sites in 15 countries.
Patients were randomized 2:1 to tolvaptan:placebo. Patients started on 45 mg in the morning and 15 mg at night and were pushed to the target dose of 90 mg in the morning and 30 mg at night as tolerated. Titration occurred for 1-3 weeks and then patients were maintained for 36 months with clinic visits every 4 months and yearly MRIs.
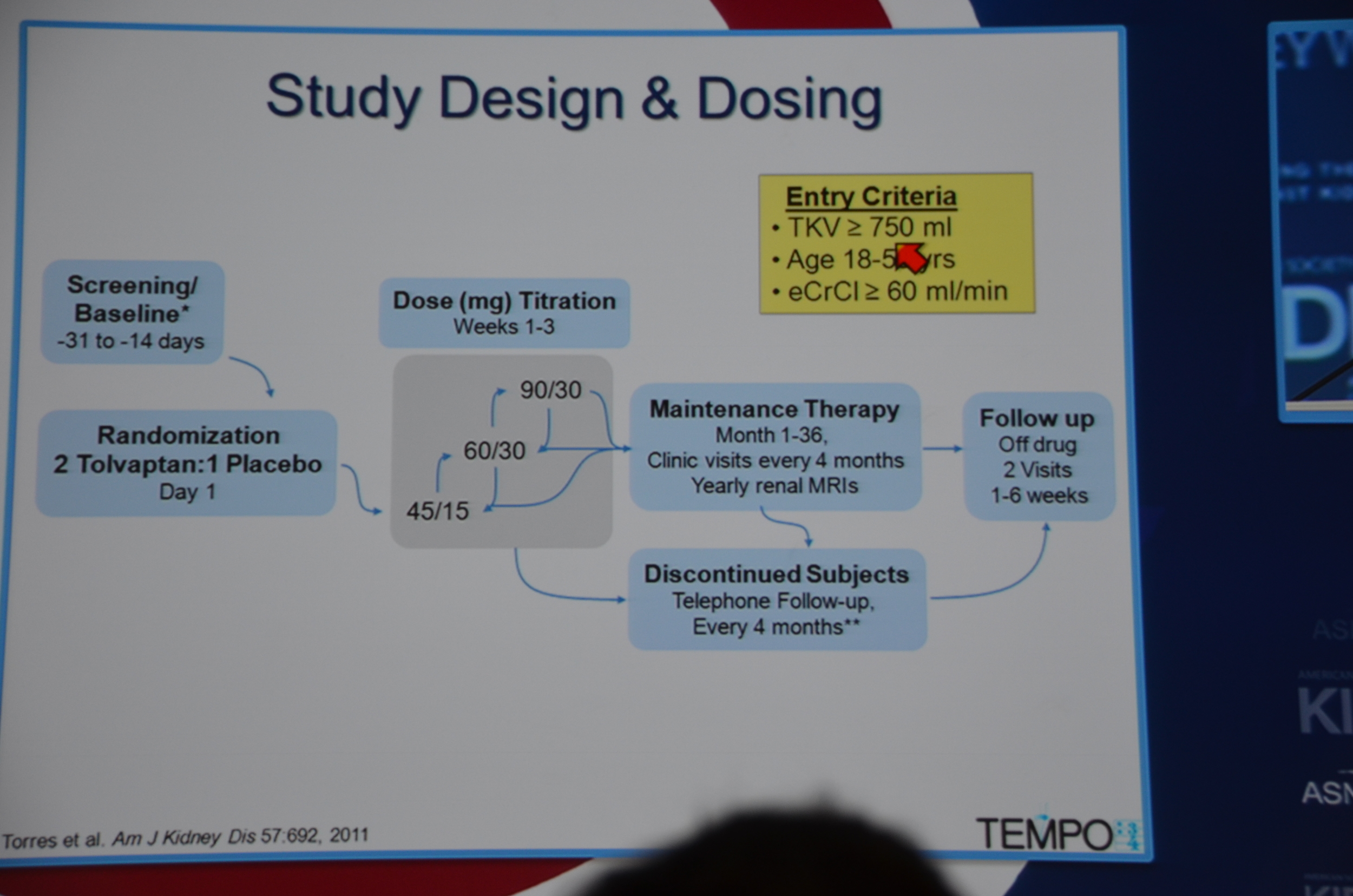
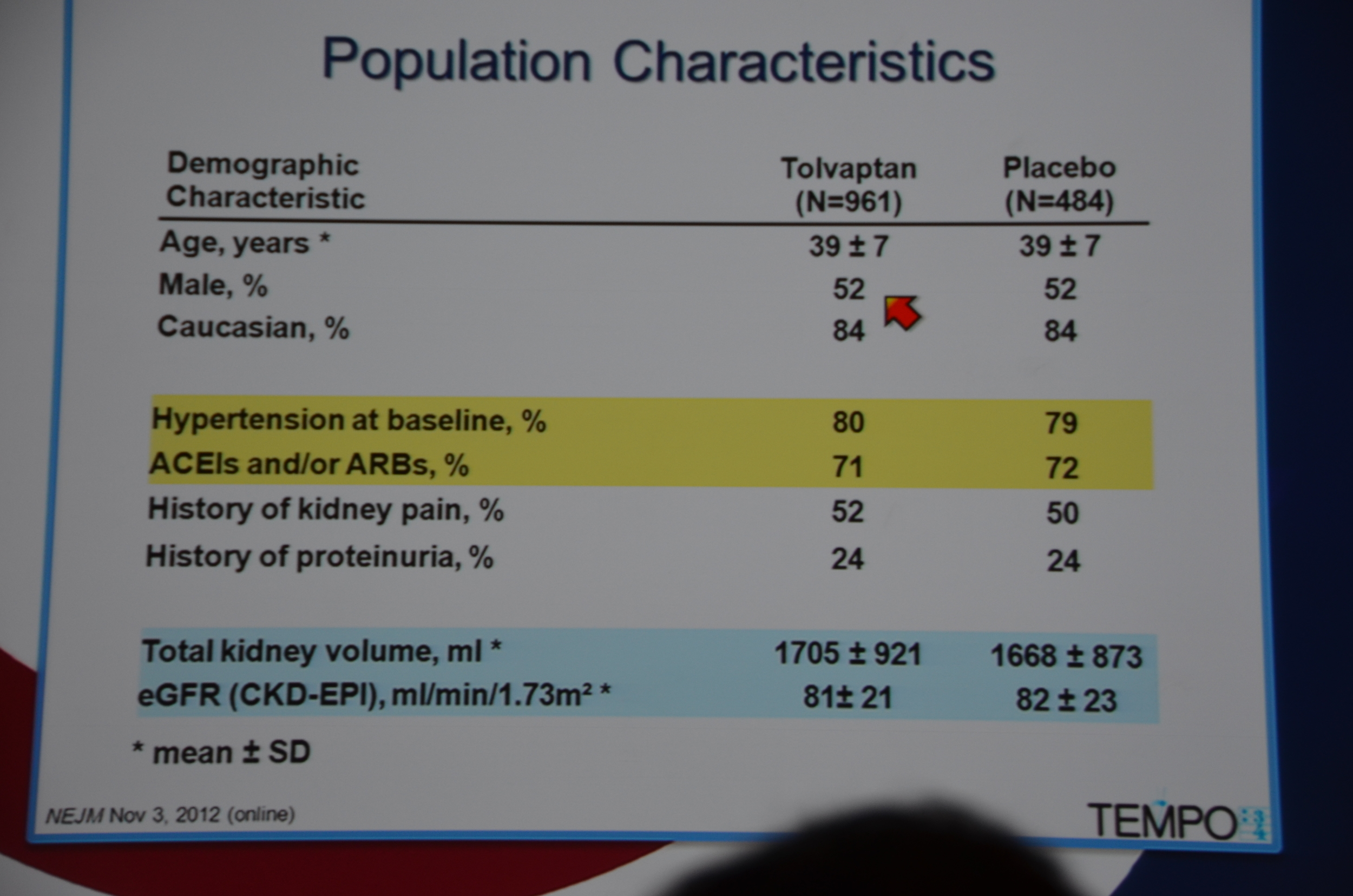
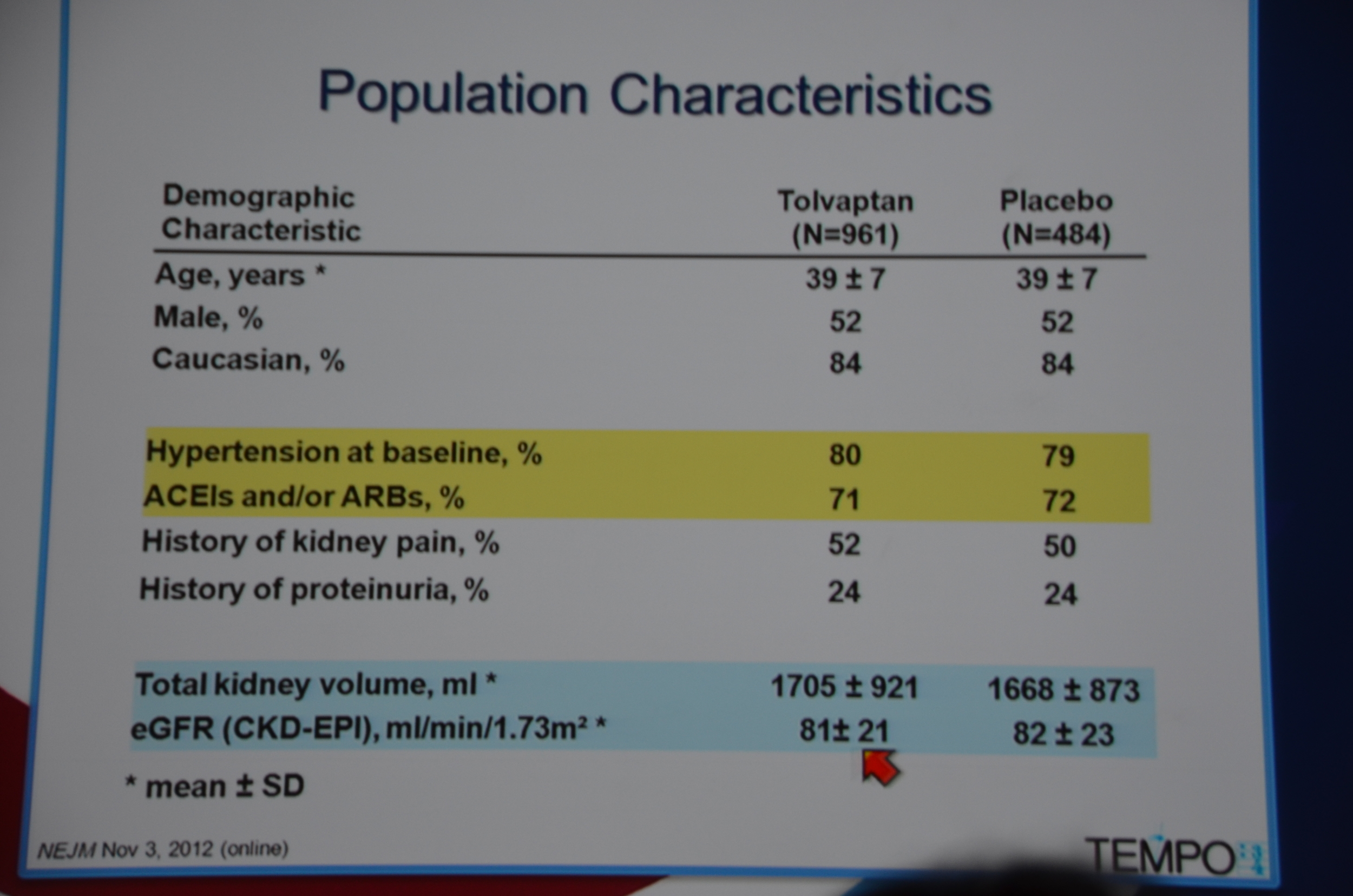
Entry criteria was a total kidney volume of 750 mL, age 18-50 years and a CrCl ≥60 mL/min.
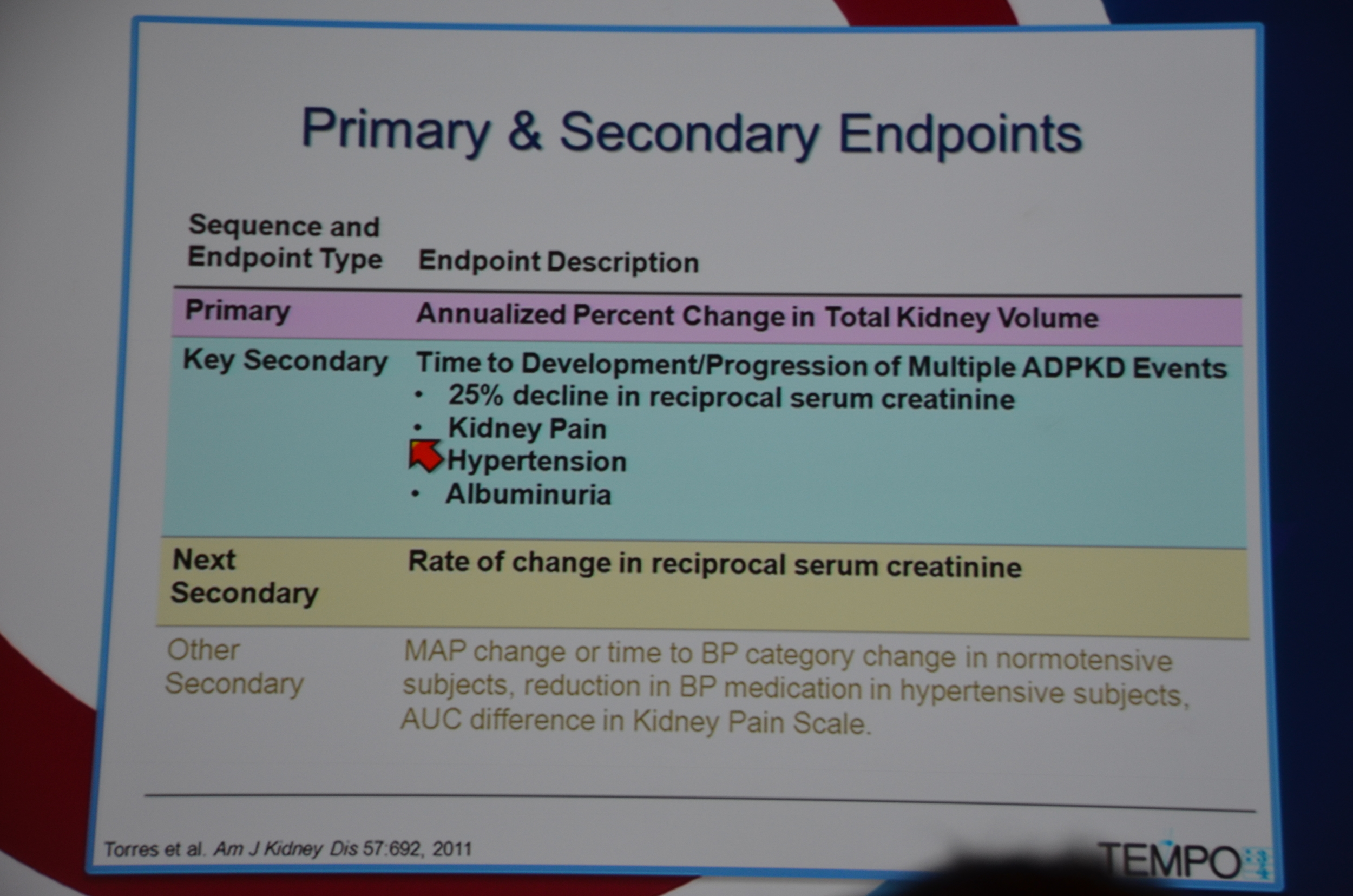
Primary endpoint was annualized percent change in kidney volume. Secondary endpoints included:
- time to 25% decline in reciprocal serum creatinine
- time to intervention for kidney pain
- time to worsening of hypertension
- time to worsening albuminuria
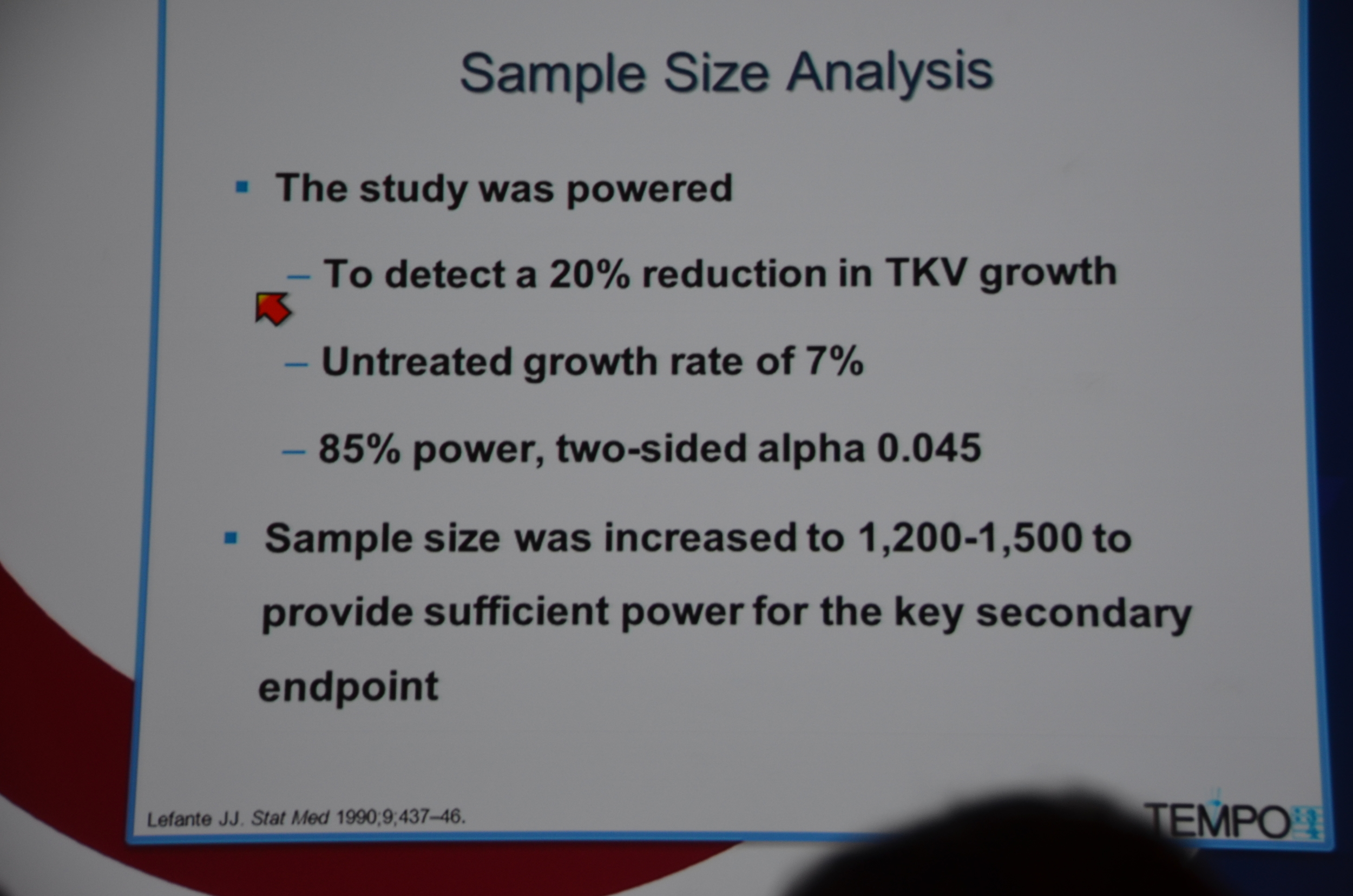
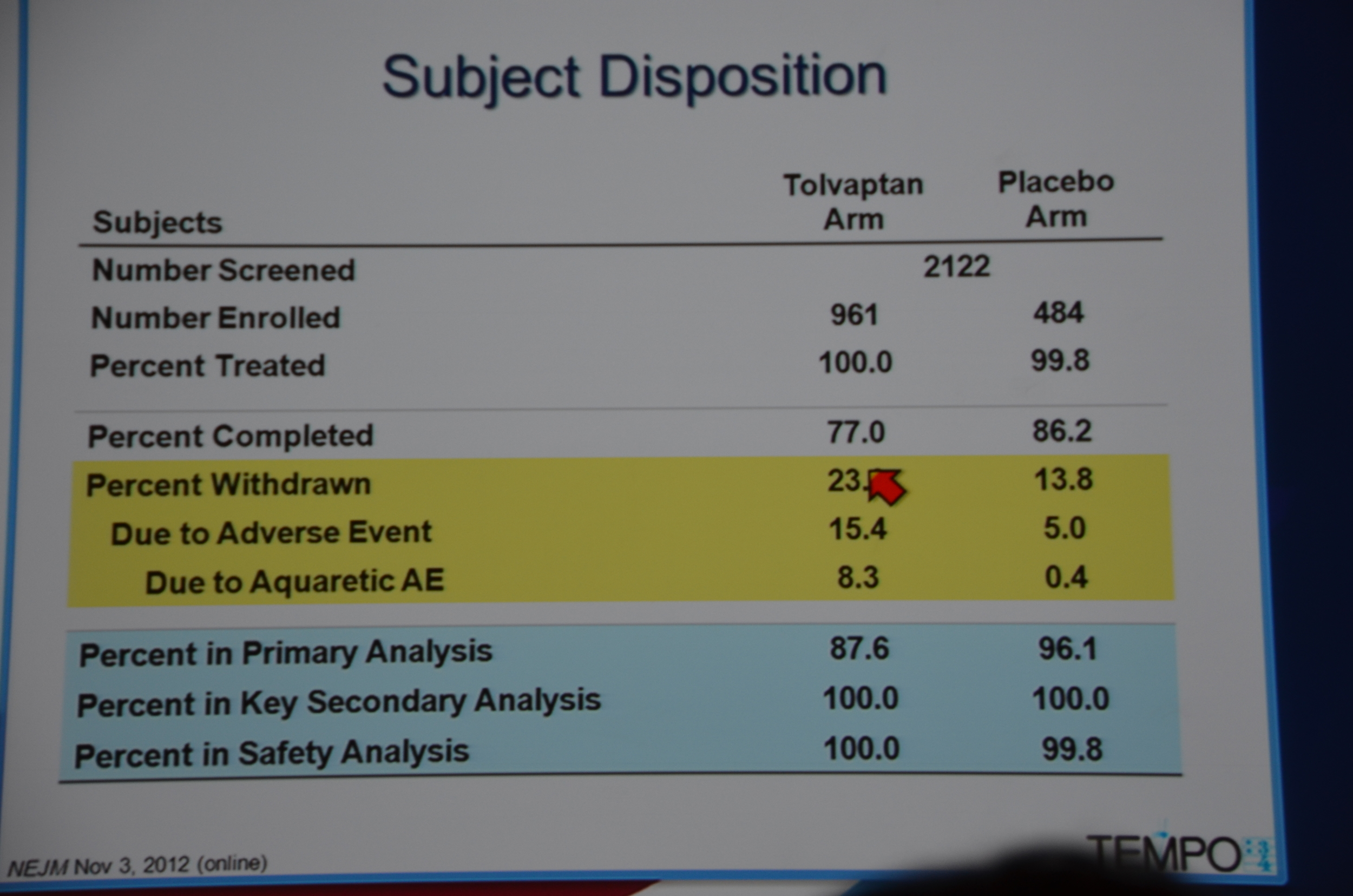
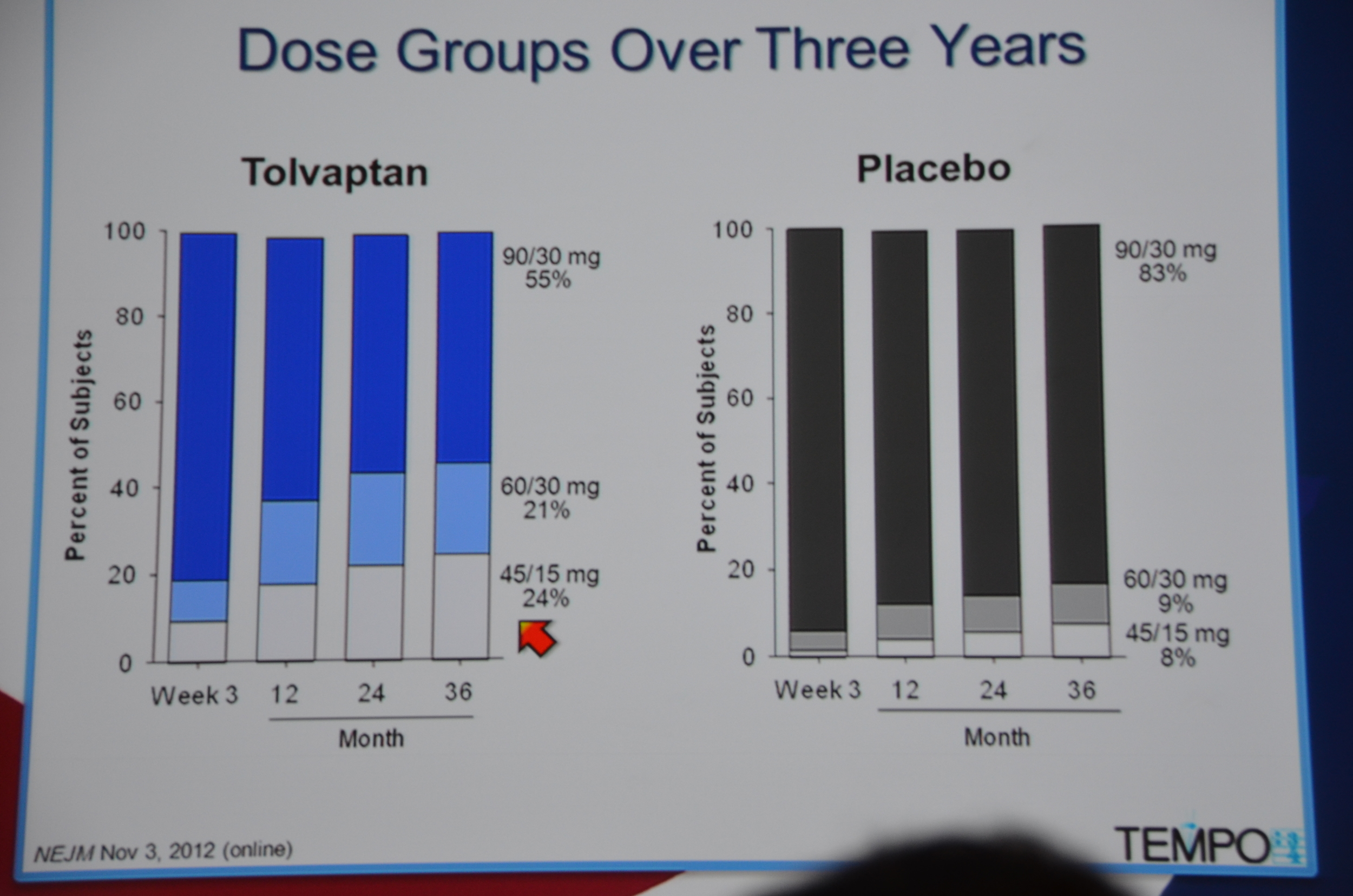
961 were randomized to Tolvaptan and 484 to placebo. Average GFR was 81 mL/min. The groups were well matched. 23% of patients on tolvaptan withdrew from the trial versus 13.8% of the placebo arm. 55% of the tolvaptan group was able to tolerate the target dose of 90 mg in the morning and 30 mg at night.
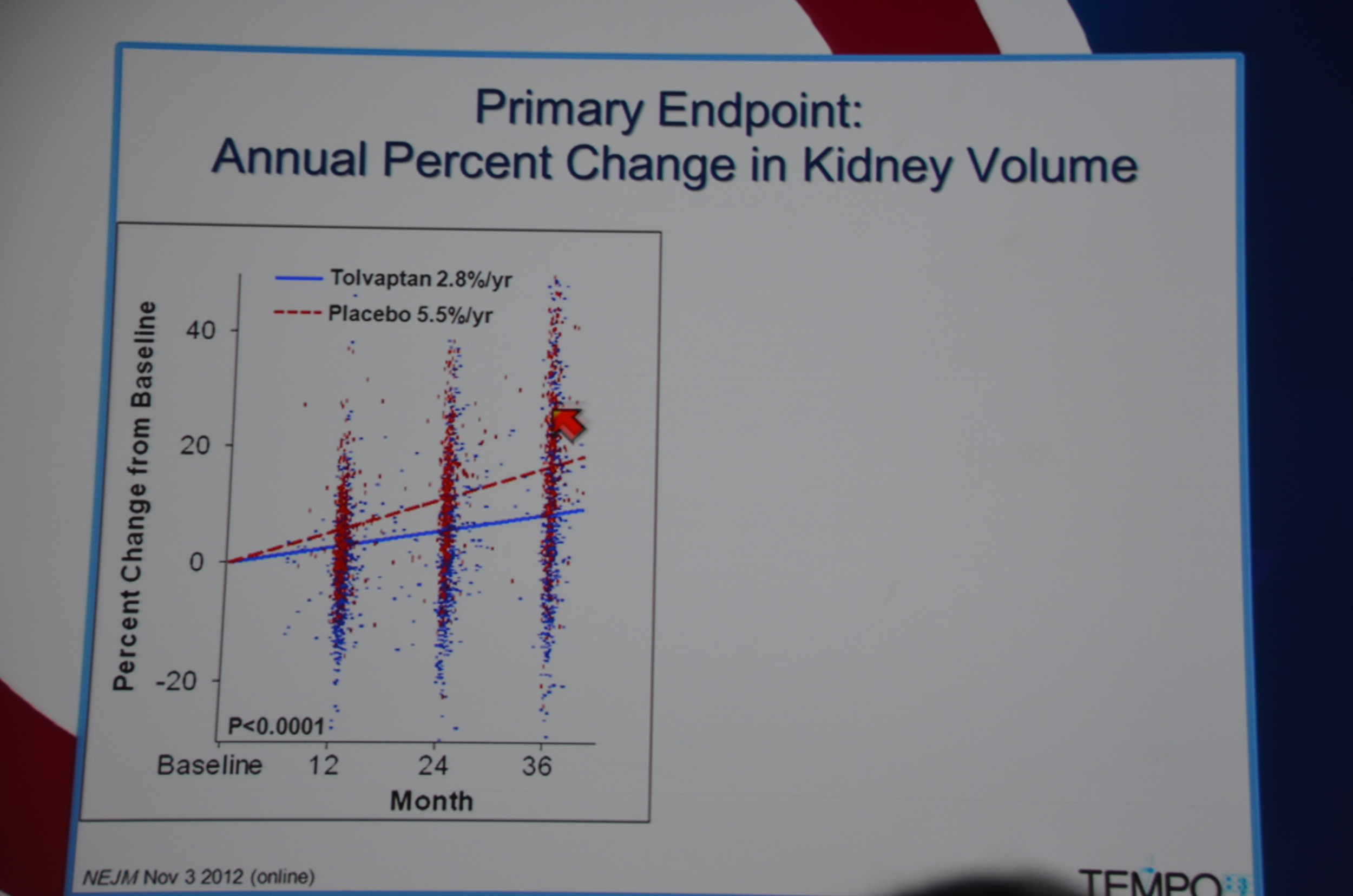
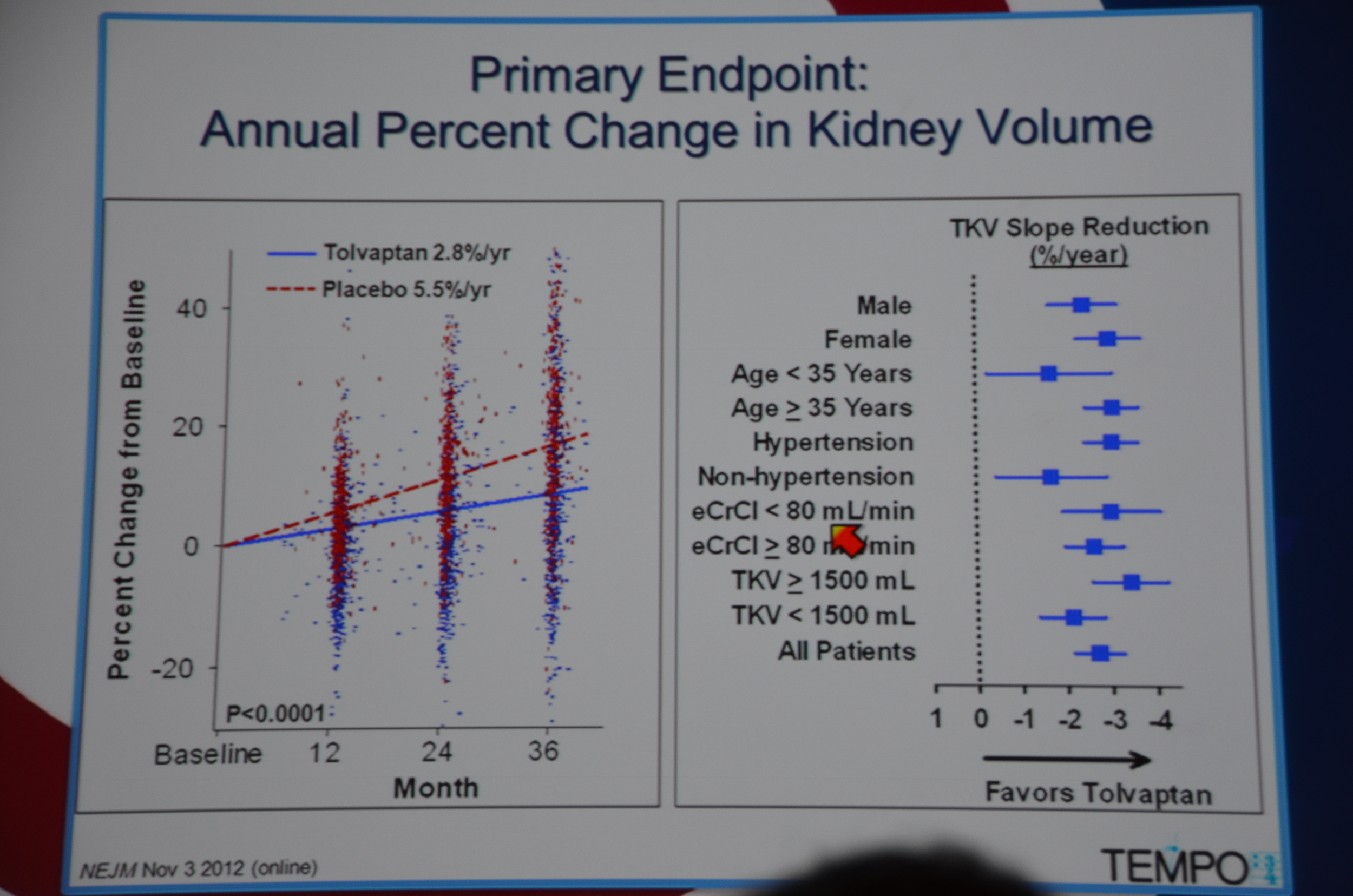
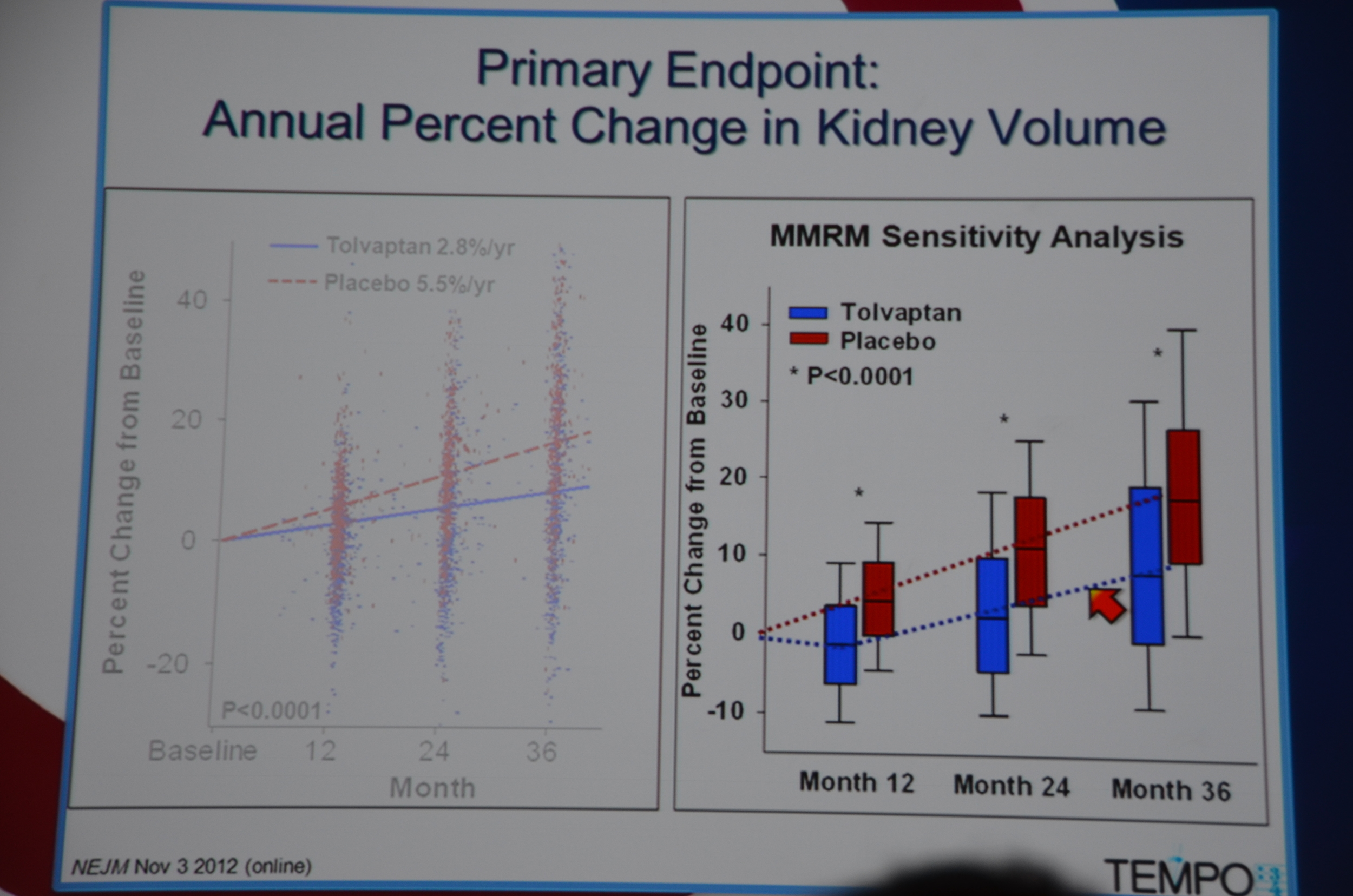
Primary end point: Tolvaptan kidney grew 2.8% a year and placebo grew 5.5% a year. They looked at 10 sub-groups and each sub-group demonstrated significant slowing of kidney growth. Win for tolvaptan.
The biggest difference was in the first year of treatment but there was continued separation of kidney sizes at 24 and 36 months too.
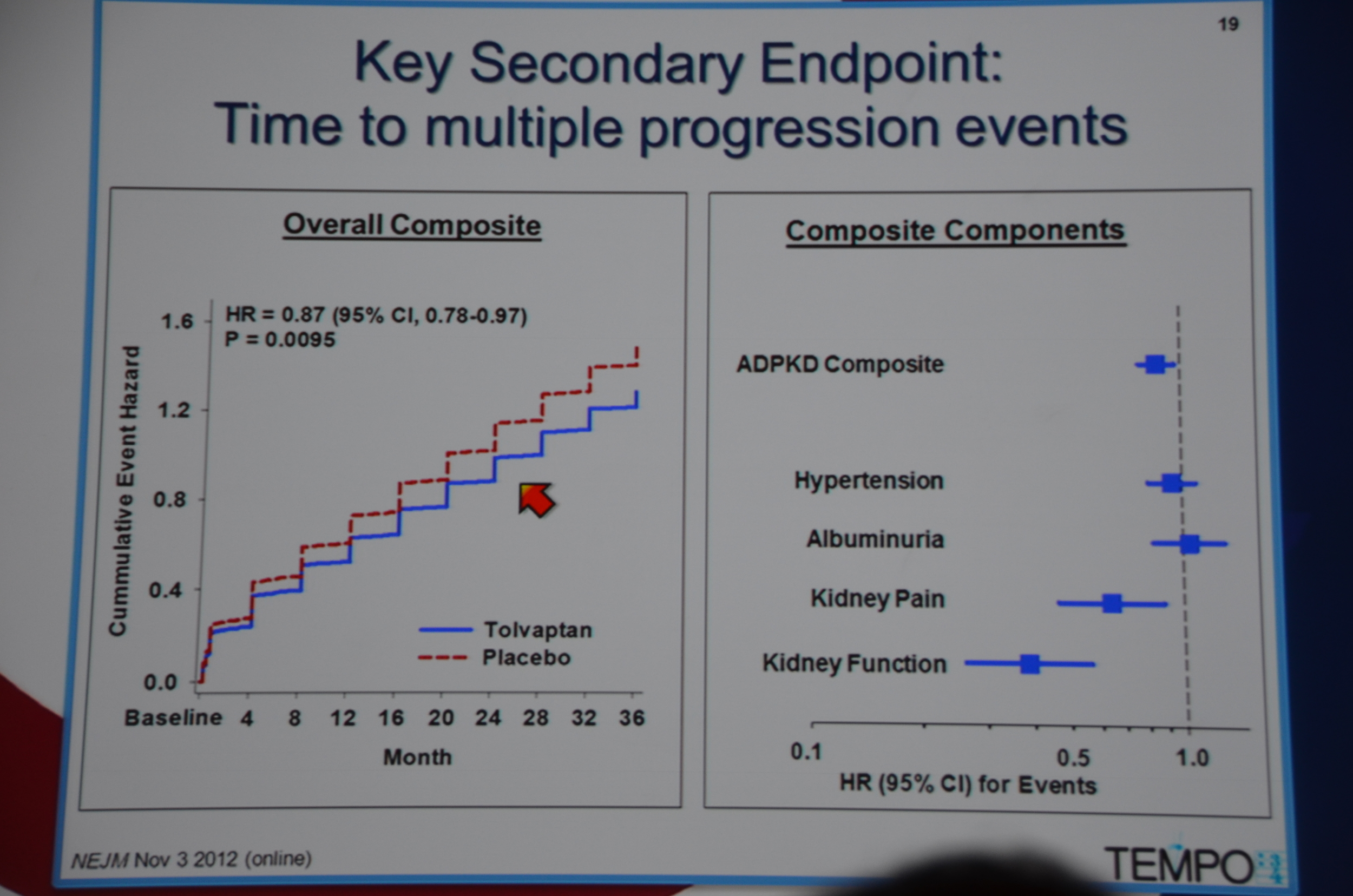
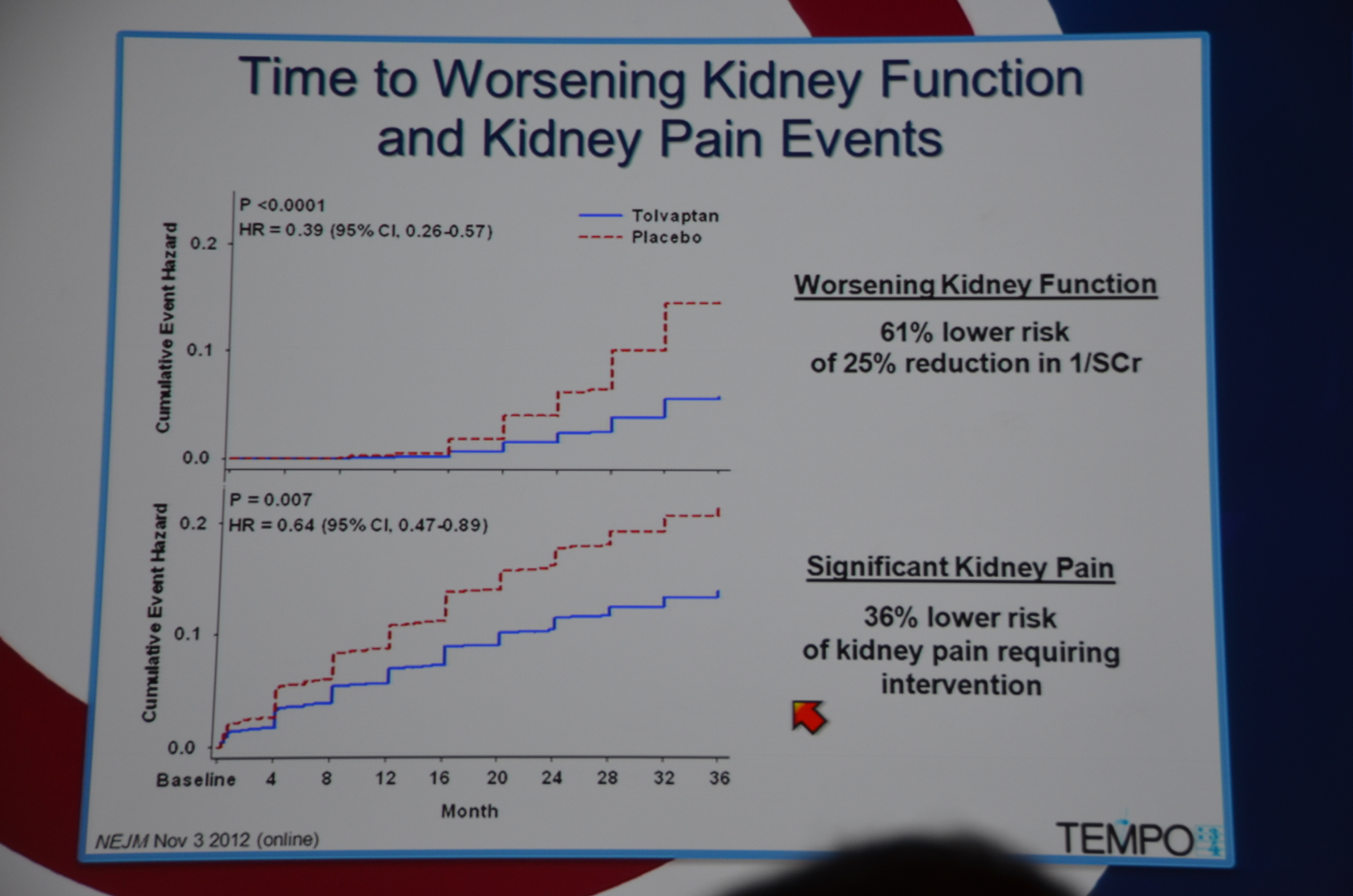
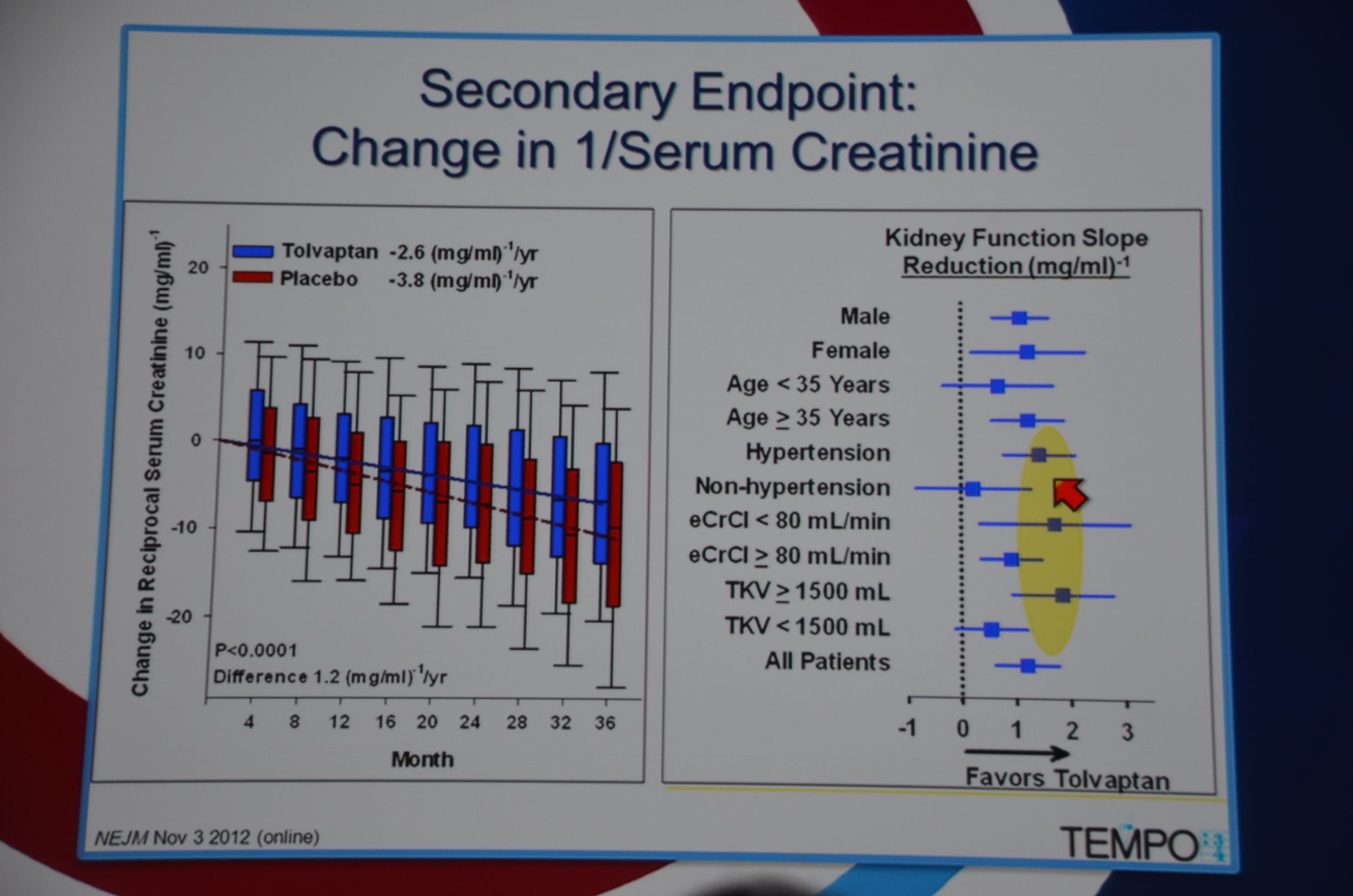
In terms of secondary end-points, there was a 61% lower risk of 1/cr decreasing 25%. The decreased loss of renal function was seen across all sub-groups, except it did not reach significance among patients without hypertension, age < 35, and kidney volume < 1,500 mL. The trends were all in the direction of benefit for tolvaptan.
36% lower risk of kidney pain requiring intervention, P=0.007.
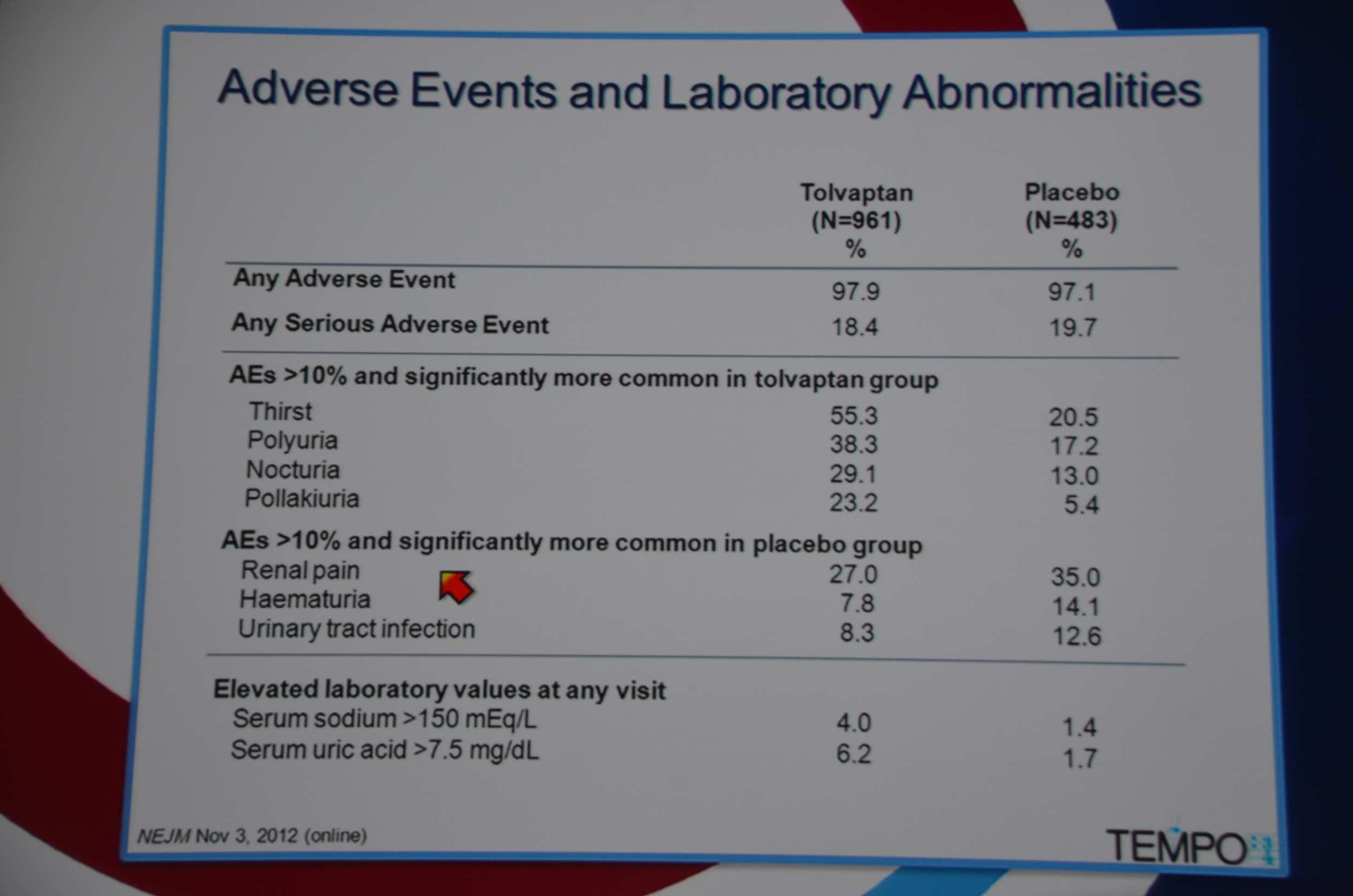
Adverse events were ubiquitous, with 97.9% of the tolvaptan group experiencing them, and 97.1% of the placebo group. Adverse events that occurred in more than 10% of the patients and were more common with tolvaptan were all related to diuresis: thirst, polyuria, nocturia and pollakiuria (frequency). The adverse events significantly more common in the placebo group included: renal pain, hematuria, and urinary tract infection.
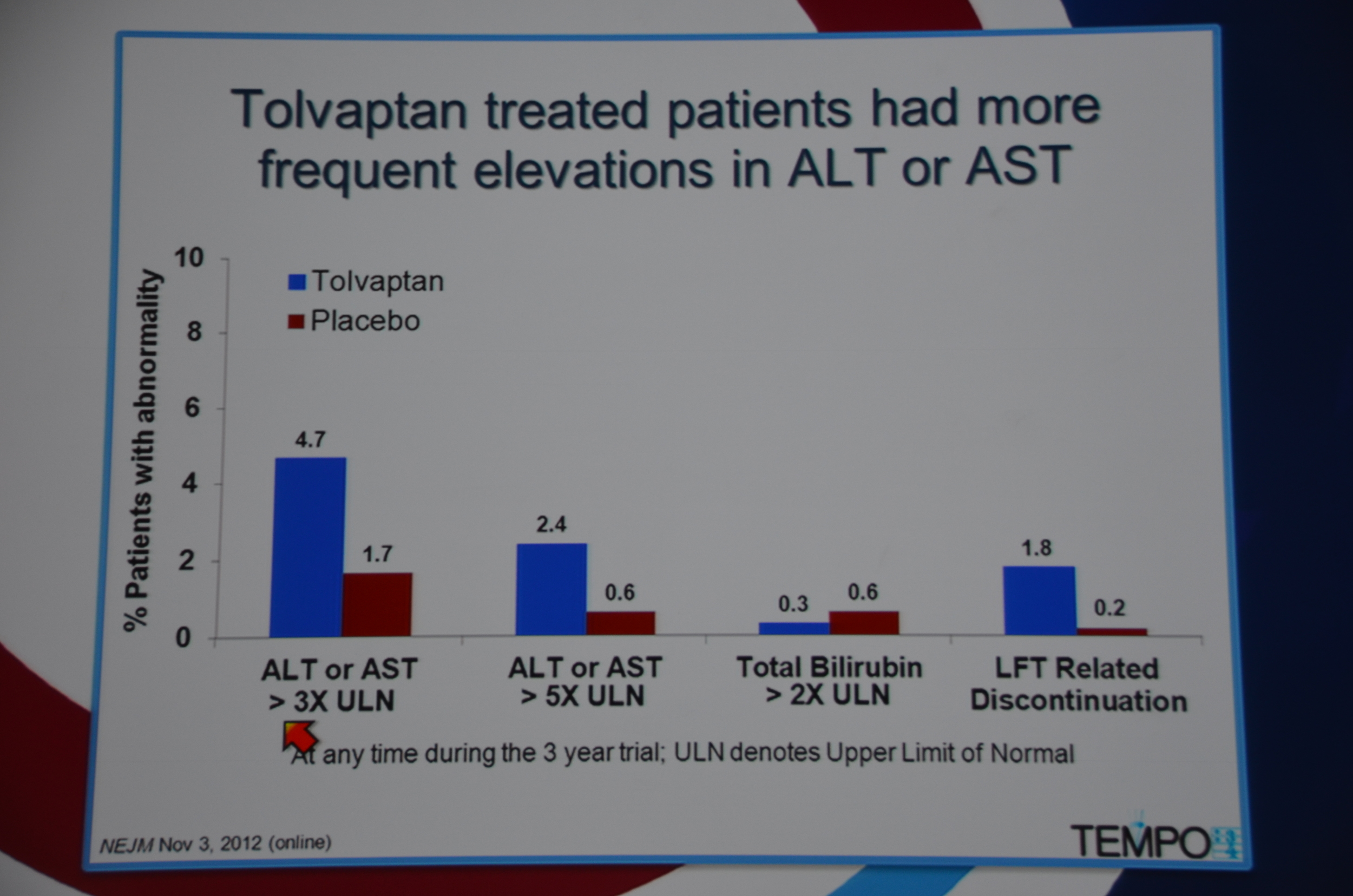
Liver enzymes were >3x the upper limits of normal in 4.7% of the patients on tolvaptan, compared to 1.7% in the placebo.
This is a major breakthrough. The first drug proven to slow cyst growth in humans. Torres pointed out that the drug is not approved for this indication and that there are many questions to be answered regarding the duration of therapy, and the optimal time to initiate therapy, but it does feel like we finally have some traction for this condition.
The study is now available at the New England Journal of Medicine website.
Post by Dr. Joel Topf, eAJKD Advisory Board Member
Here is my live tweet stream of the event:
eAJKD Torres from Mayo with Tolvaptan for ADPKD Tempo 3/4 trial #KidneyWk12
eAJKD Vasopressin stimulates cystogenesis #KidneyWk12
eAJKD Preclinical trials show blocking vasopressin reduces cysts. #KidneyWk12
eAJKD phase 3, randomized, multi center, placebo controlled, double blind #KidneyWk12
eAJKD high doses up to 90 mg AM 30 mg PM #KidneyWk12
eAJKD Had to have large kidney but preserved renal function GFR>60 to enroll. 1400 patients randomized endpoint change in kidney vol #KidneyWk12
eAJKD 23% on tolvaptan stopped the drug due to adverse event or aquatic adverse event (AE while swimming?) #KidneyWk12
eAJKD 55% were able to tolerate the crazy high dose. #KidneyWk12
eAJKD 2.8% fgrowth in tolvaptan vs 5.5% in control. I smell a positive trial! #KidneyWk12
eAJKD Most effect in the first year. Increasing separation during trial #KidneyWk12
eAJKD Improved KIDNEY FUNCTION AND DECREASED PAIN! #KidneyWk12
eAJKD 61% reduced risk of increased cr of 25% #KidneyWk12
eAJKD More effect in patients with more severe disease! #KidneyWk12
eAJKD RT @UKidney: A major breakthrough in #PCKD treatment is likely upon us. #KidneyWk12 #pkd #tolvaptan #KidneyWk12
eAJKD 5% had increased liver enzymes 8% had aquaresis limit use of drug #KidneyWk12
eAJKD Patients were on 4 30 mg pills a day. Last I checked these were $250/pill so $1,000 a day. #KidneyWk12
eAJKD Hopefully this leads to FDA approval of new indication and then insurance coverage. #KidneyWk12
eAJKD Question who should be treated and how long, investigator says that is premature, asking for patience until FDA review. #KidneyWk12
PDF from the NEJM

Cincalcet for Hyperparathyroidism
Cincalcet for Hyperparathyroidism
“Why was the primary result of the trial negative? The surprising imbalance in baseline characteristics between the two groups may well have had an effect — and probably represents simple bad luck but illustrates the importance of stratification for key prognostic factors. More important were the high rates of treatment crossover during the trial: almost two thirds of patients in the cinacalcet group discontinued active therapy, and one fifth of those in the placebo group started taking commercially available cinacalcet before trial completion. The resultant reduction in the between-group separation in parathyroid hormone levels substantially reduced the power of the trial to test its hypotheses.”




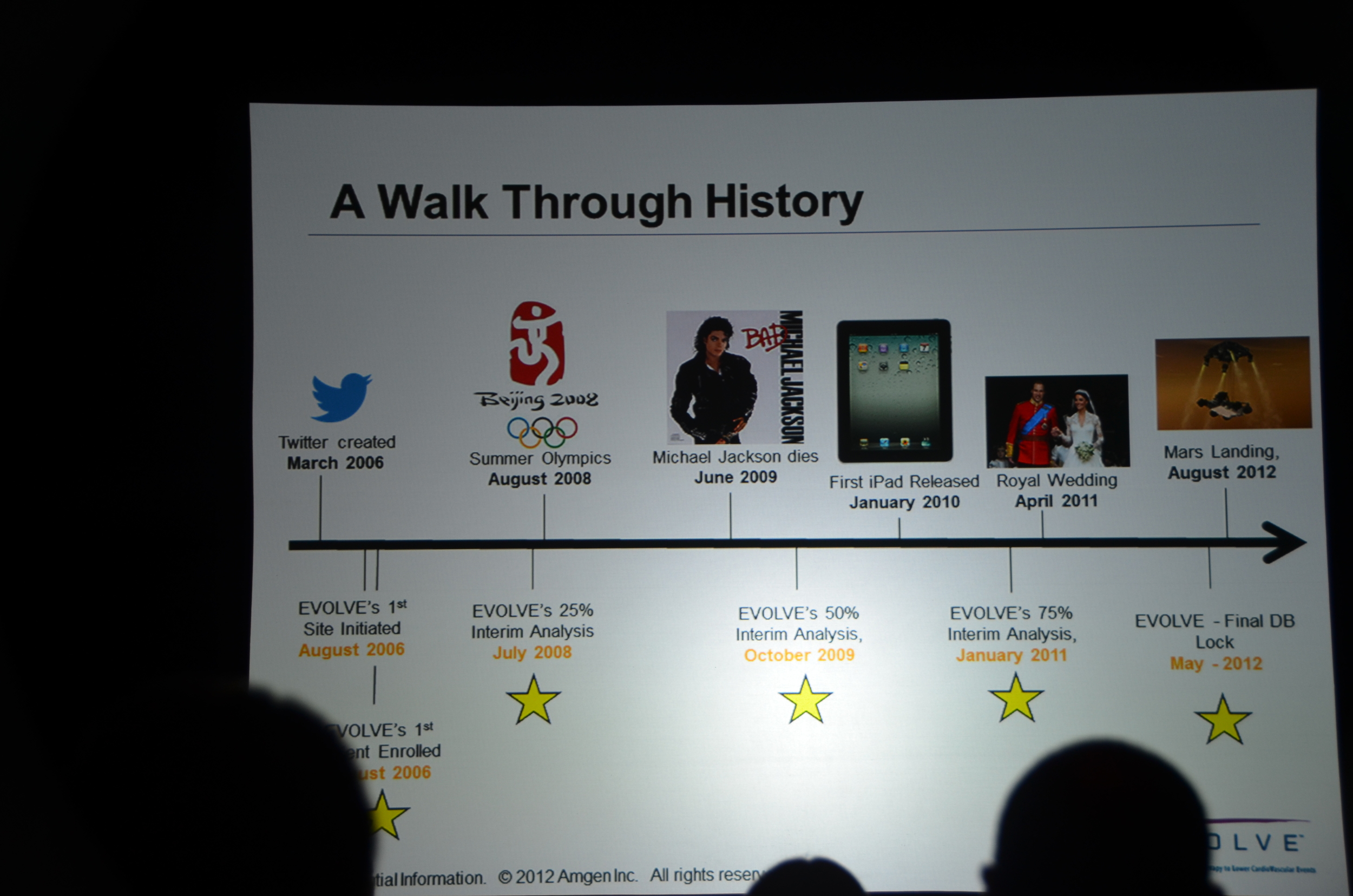
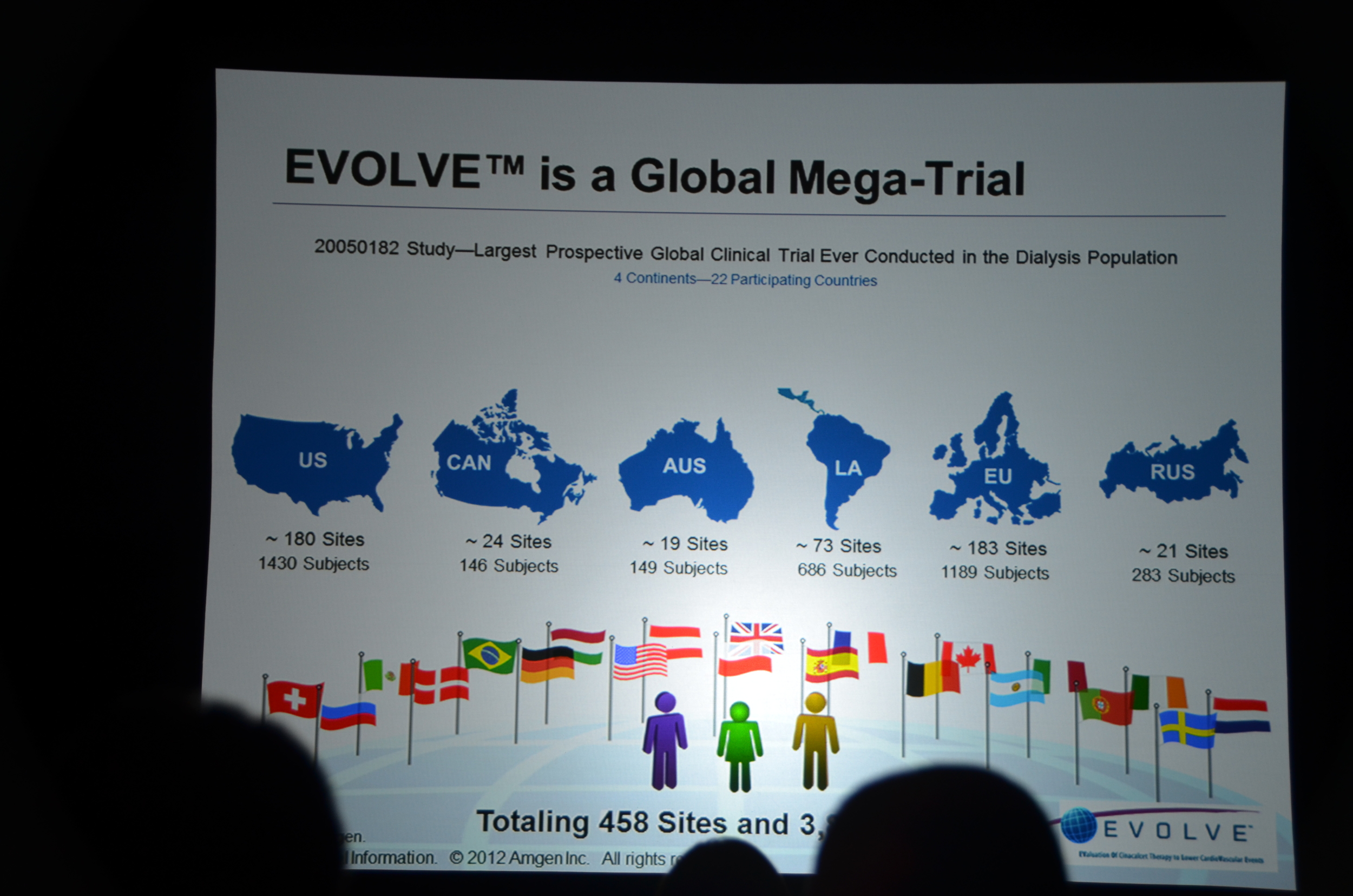

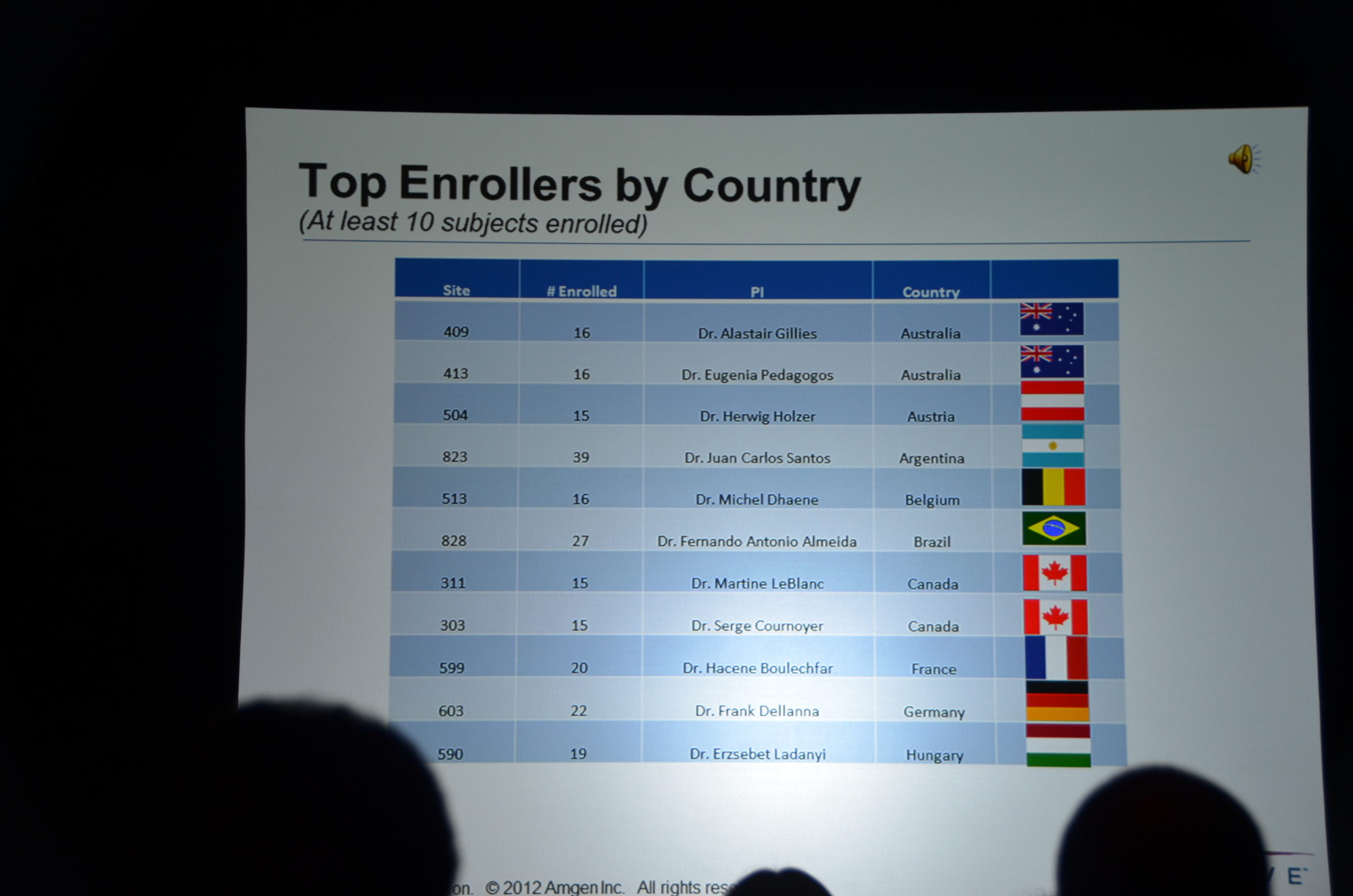







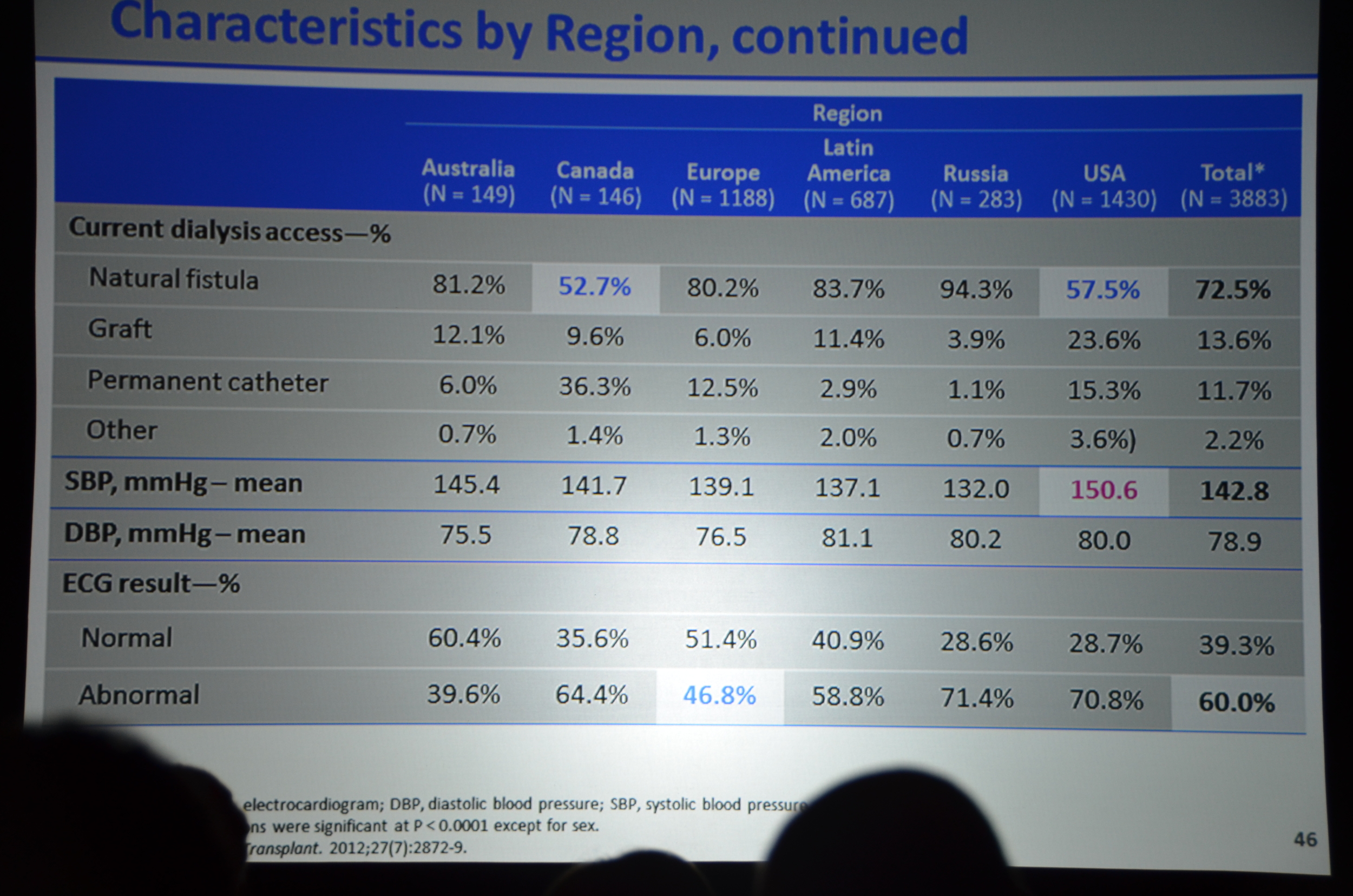
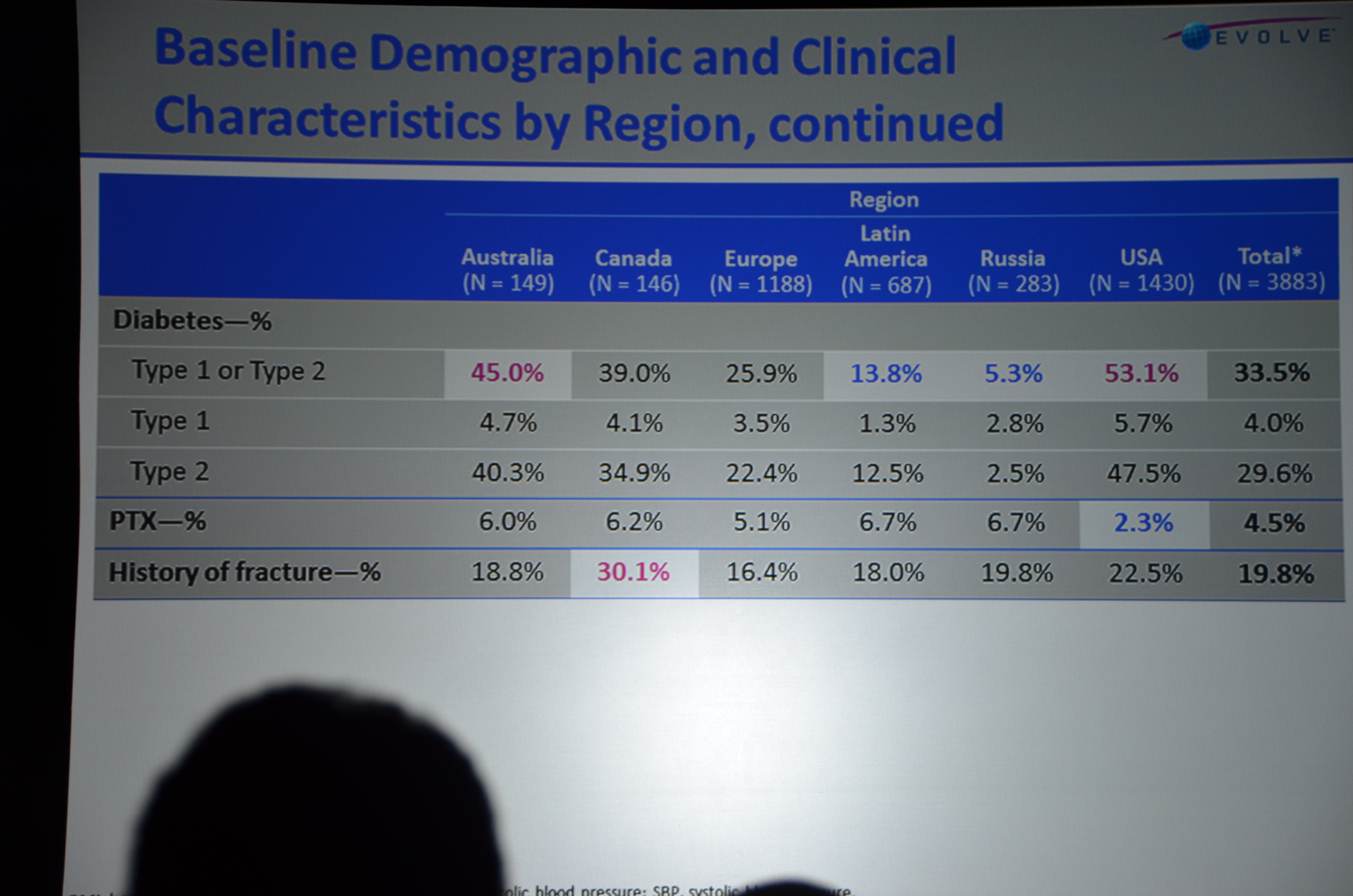
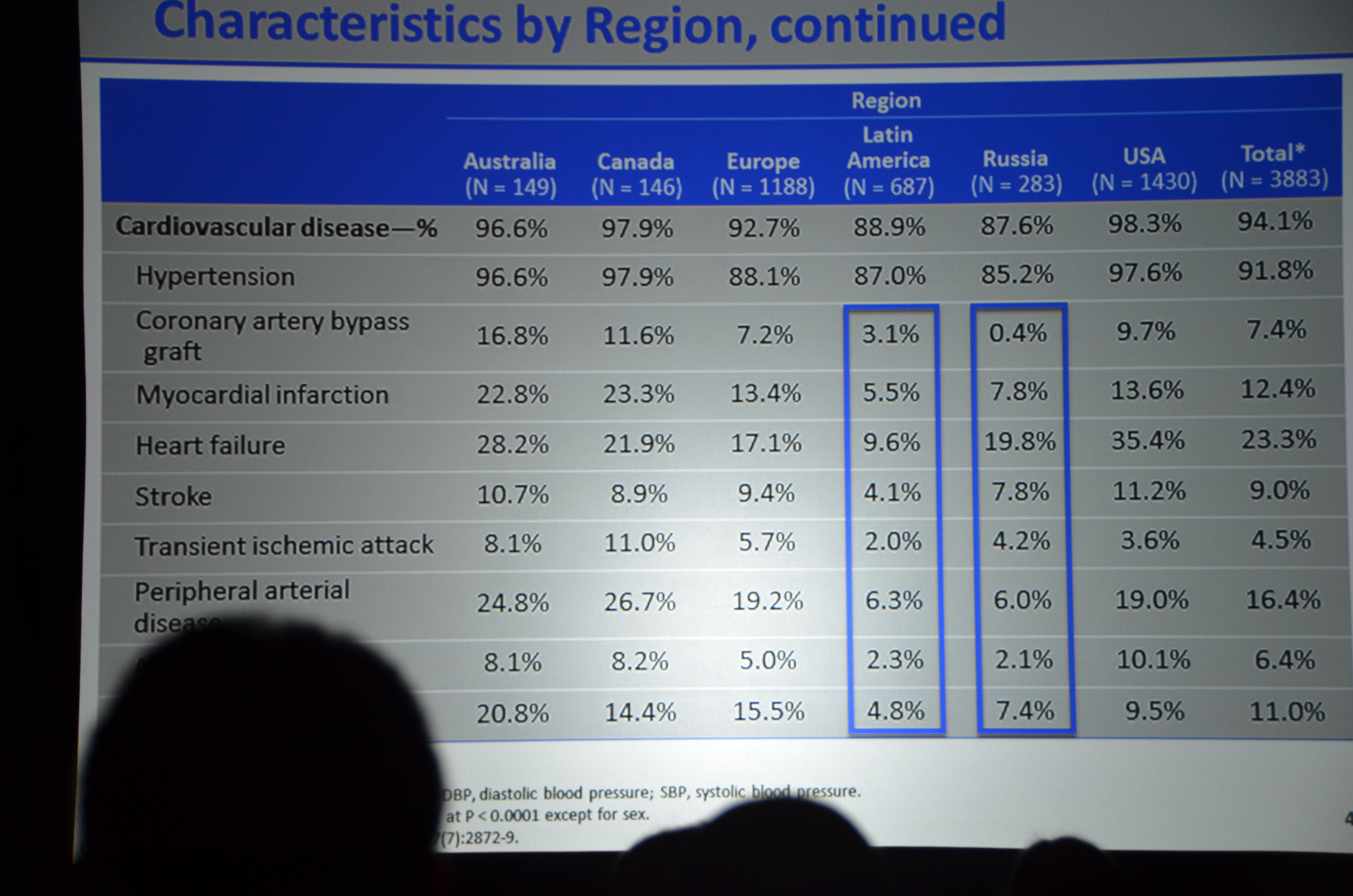
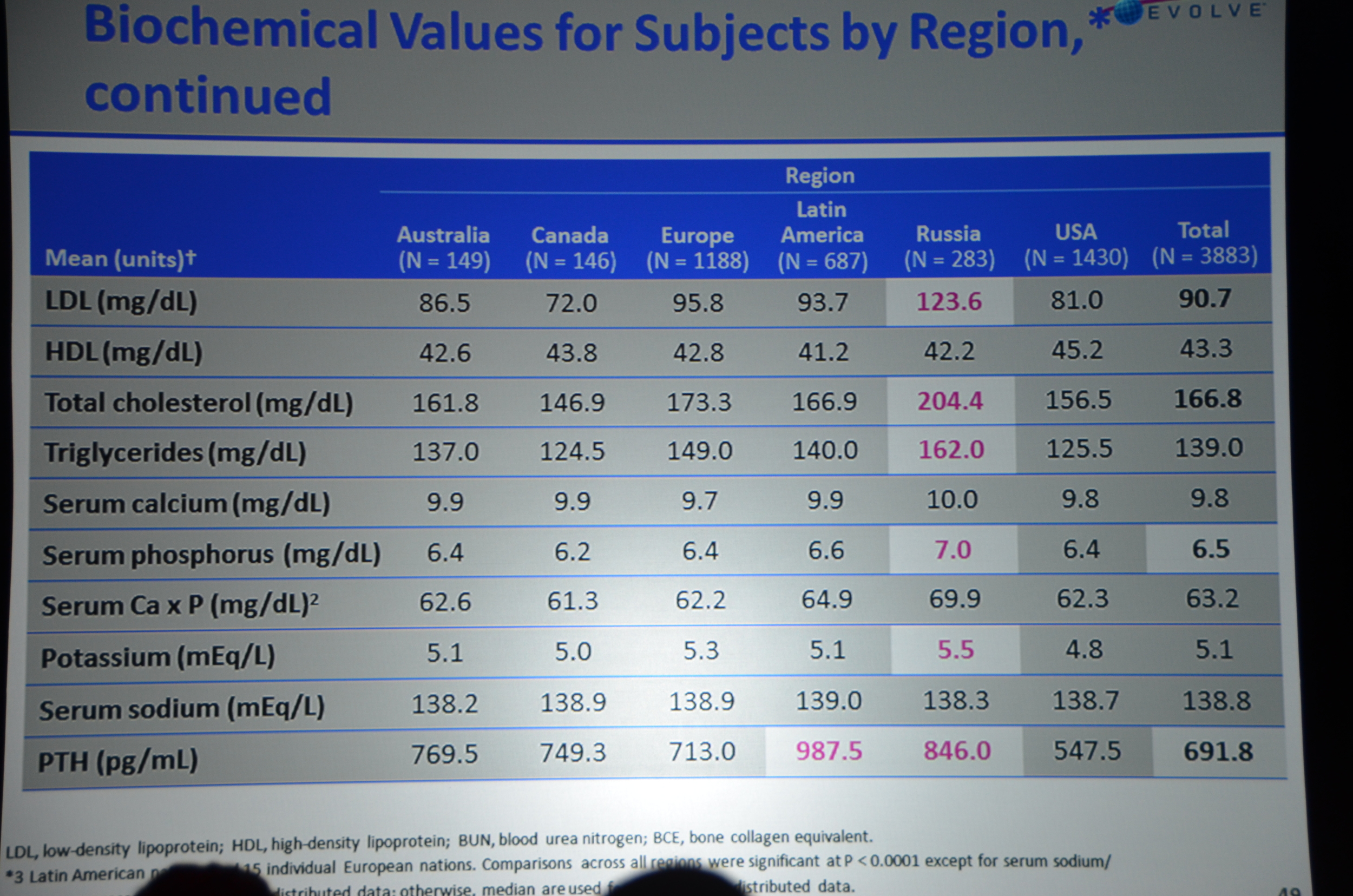
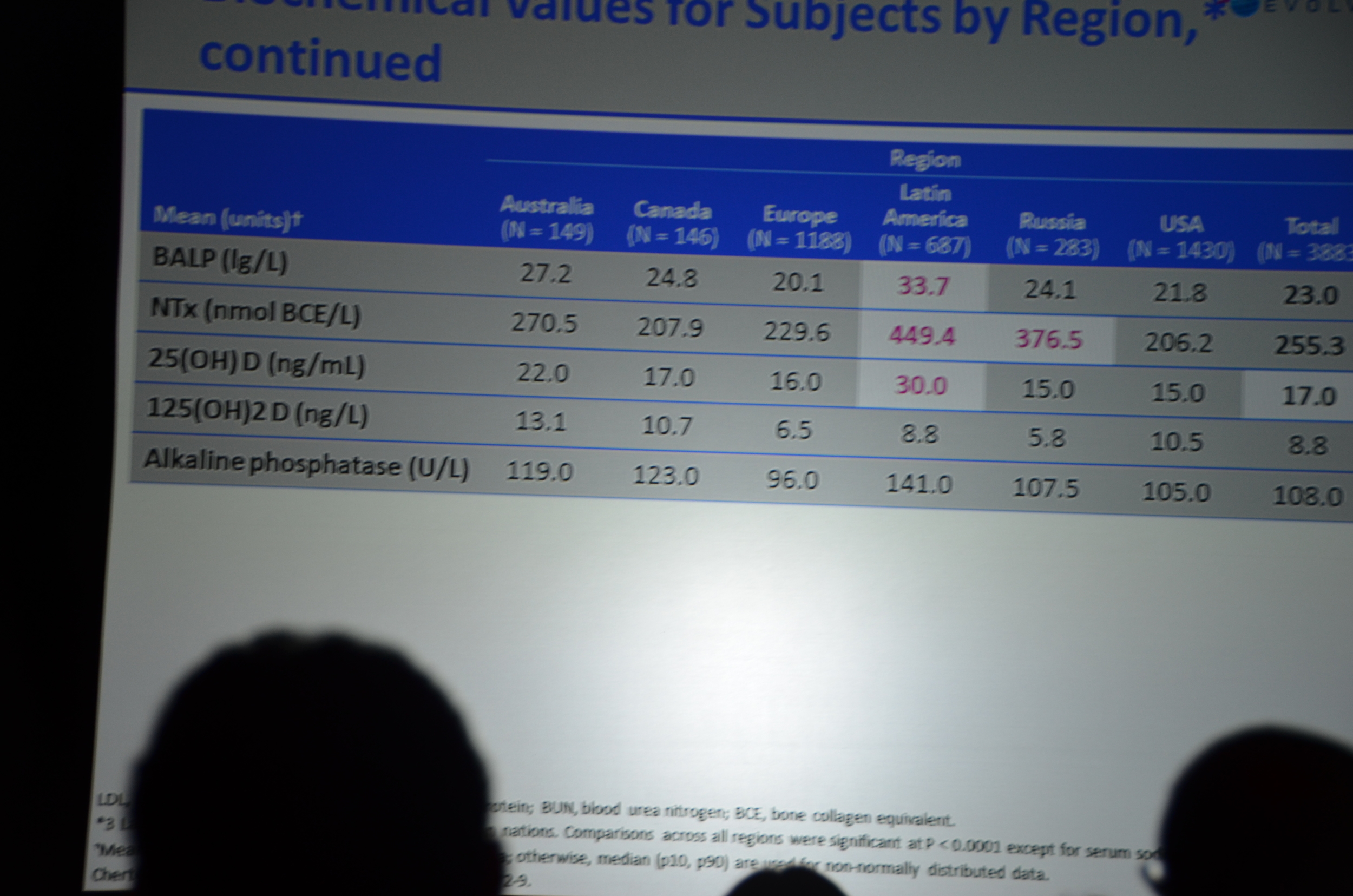
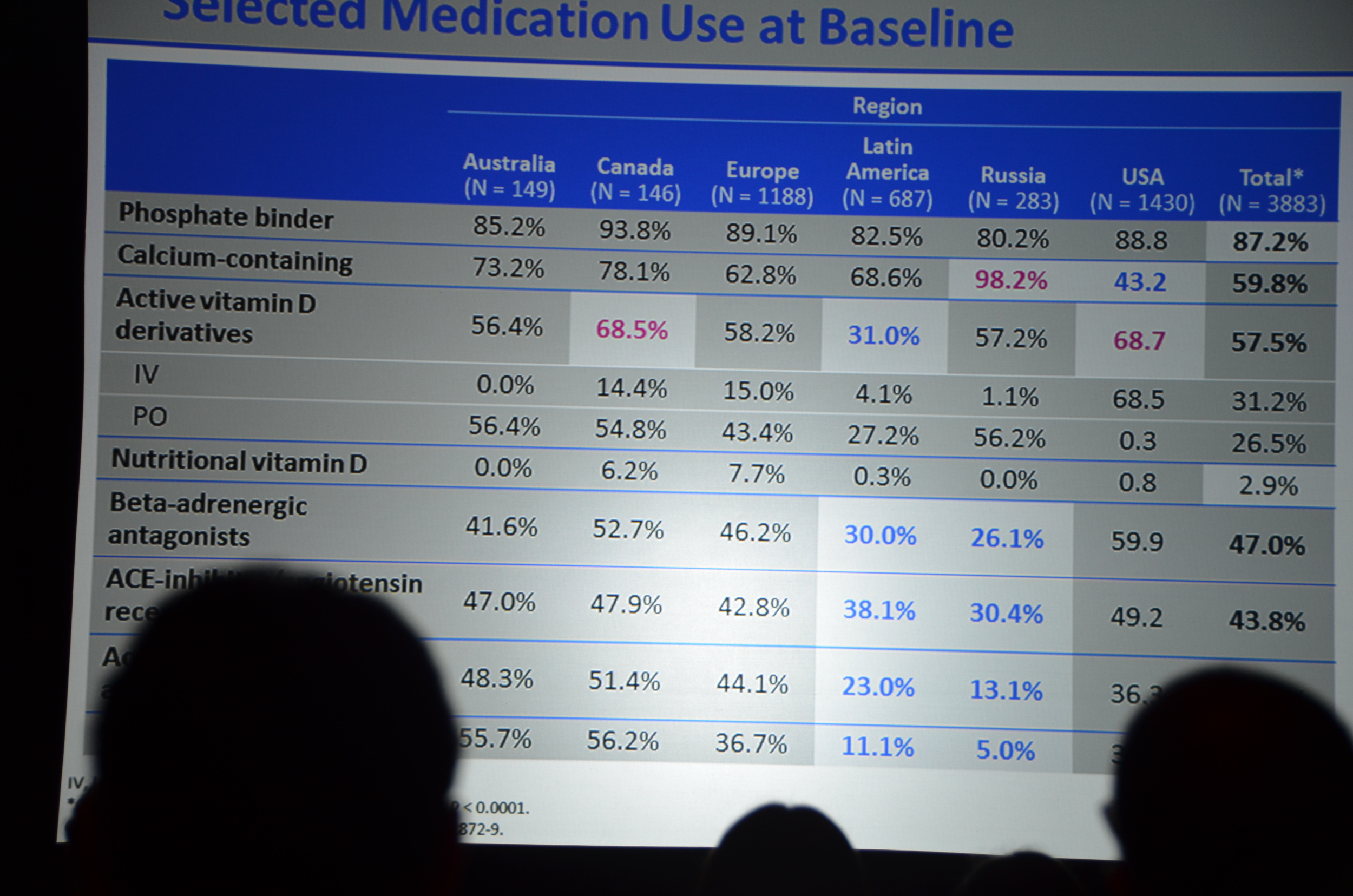
Here is what I wrote for eAJKD
Kidney Week 2012: Late Breaking Trials: EVOLVE
November 4, 2012 By
The cinacalcet randomized controlled trial: EVOLVE.
Speaking is the lead author Glen Chertow.
Disclosure: I (Joel Topf) was a primary investigator for EVOLVE.
The second trial to be presented at late breaking and high-impact trials is EVOLVE.
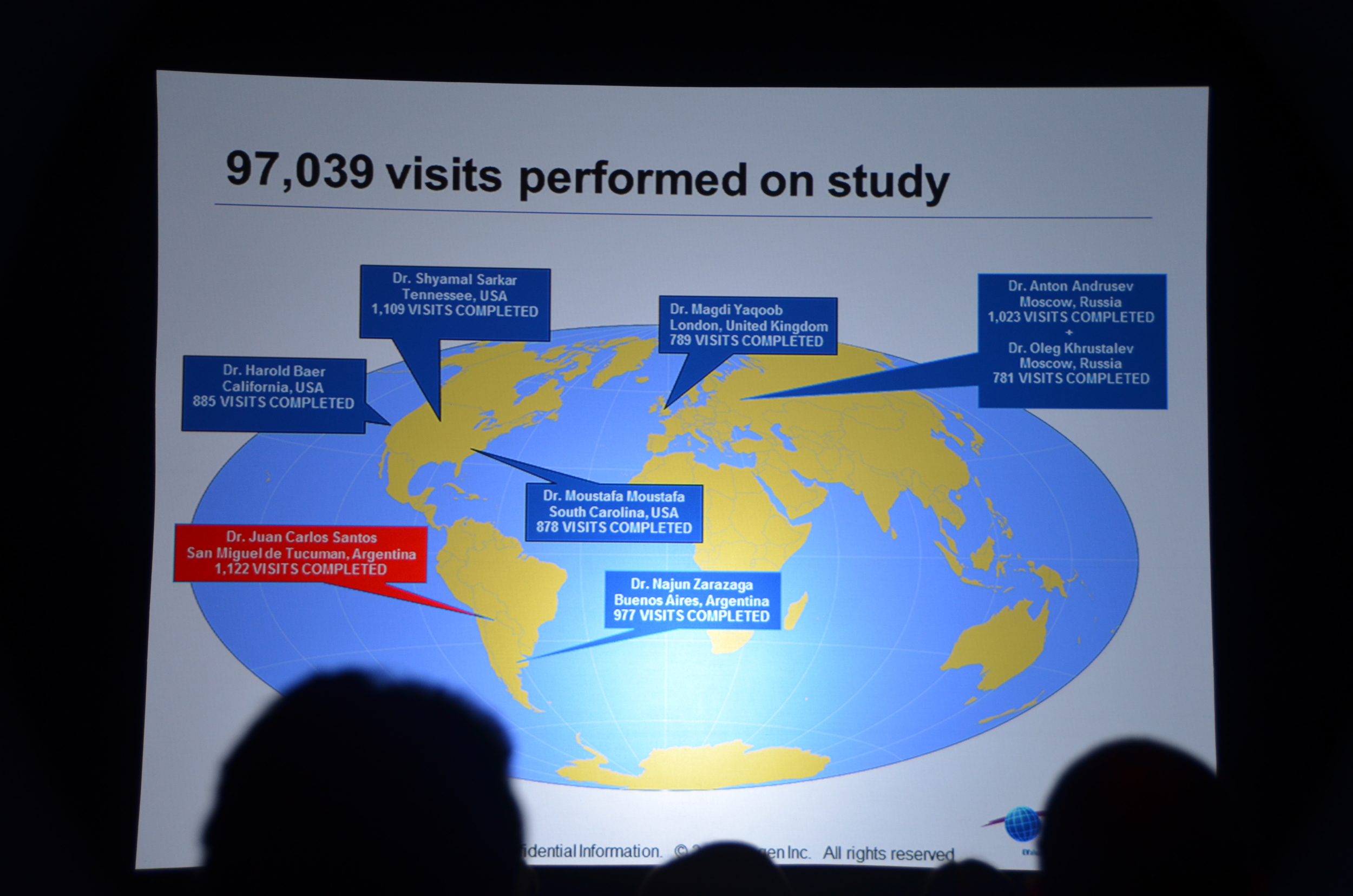
- Largest dialysis study
- Longest dialysis study
- Most international dialysis study
- Only double-blind dialysis study
- If you complain that nephrology does not have enough high quality studies you should pay attention to EVOLVE.
Cinacalcet was approved based on its ability to lower the PTH, the next question was, “does lowering the PTH with cinacalcet reduce mortality?” This was an investigator originated study that was sponsored by Amgen.
Dr. Chertow showed a slide with the hypothesis: “EVOLVE was designed to test the hypothesis that treatment with cinacalcet would reduce the risks of death and nonfatal cardiovascular events among patients with secondary hyperparathyroidism who were undergoing hemogdialysis.”
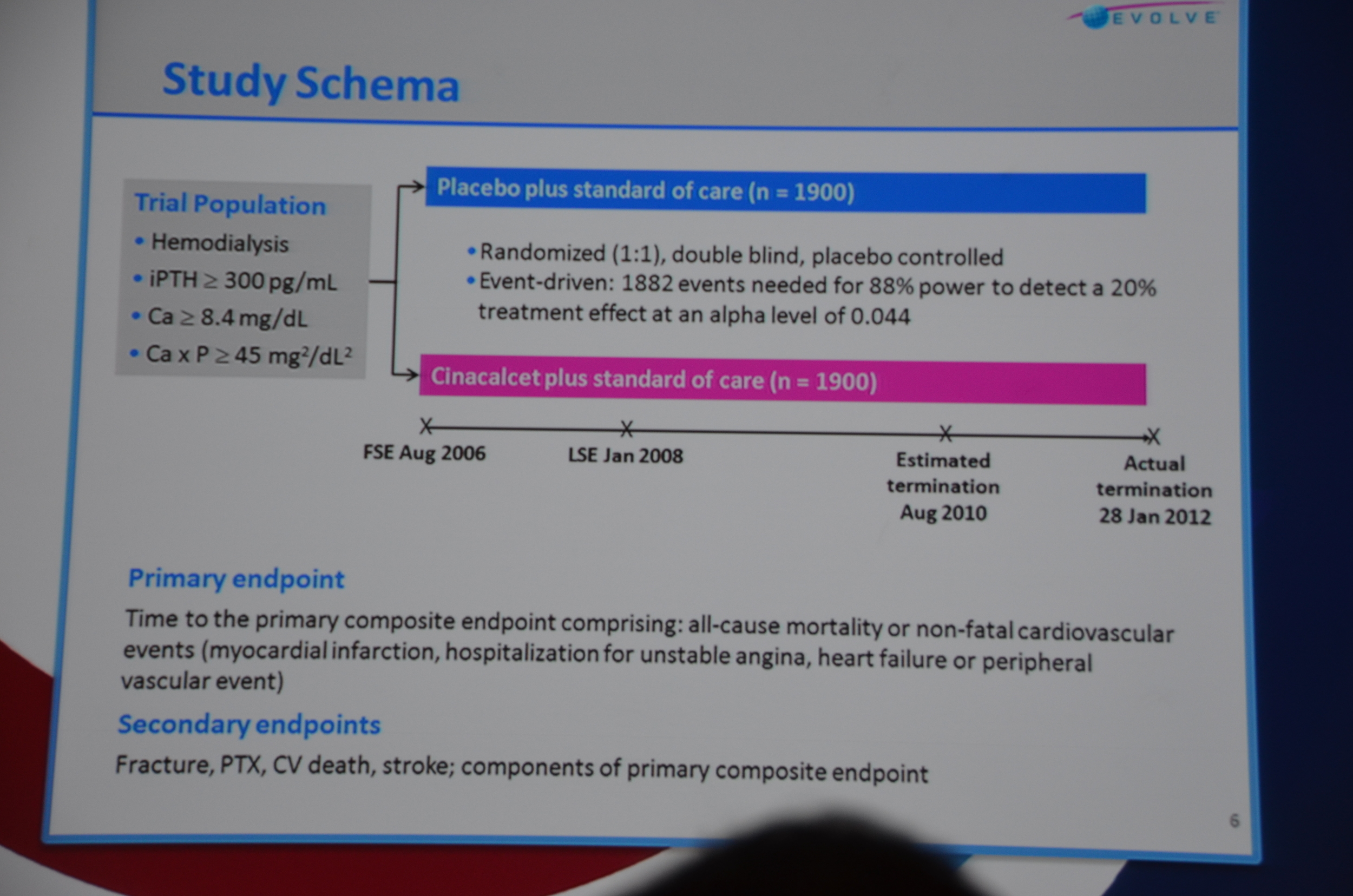
Patients were randomized 1:1 to cinacalcet or matching placebo. The trial was event driven, i.e., they ran the trial until they had 1,882 end-points. This was projected to take 3 years. It took 5.
The primary endpoint was a composite of all-cause mortality, or non-fatal cardiovascular event (MI, hospitalization for unstable angina, heart failure, or peripheral vascular event).
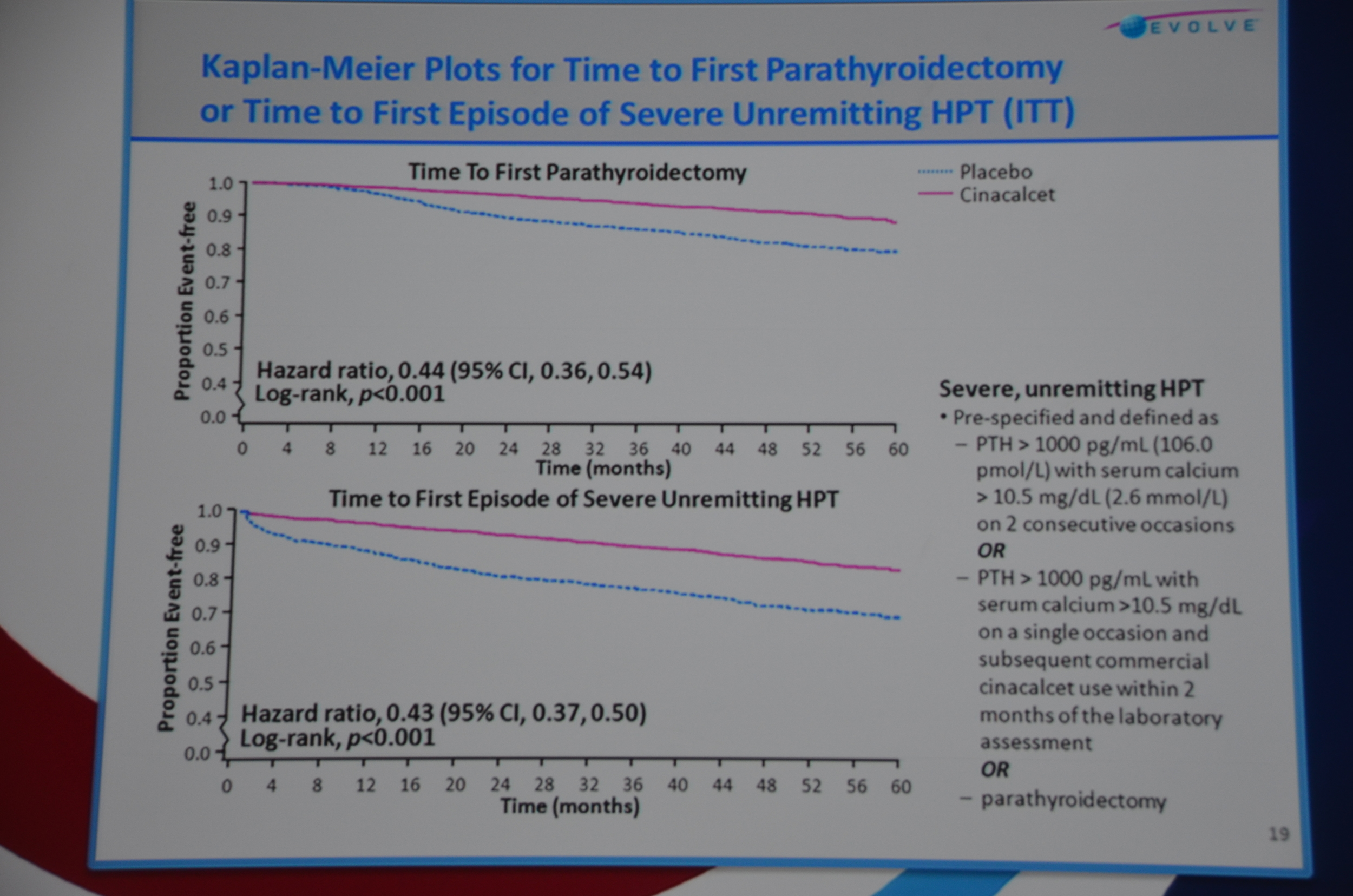
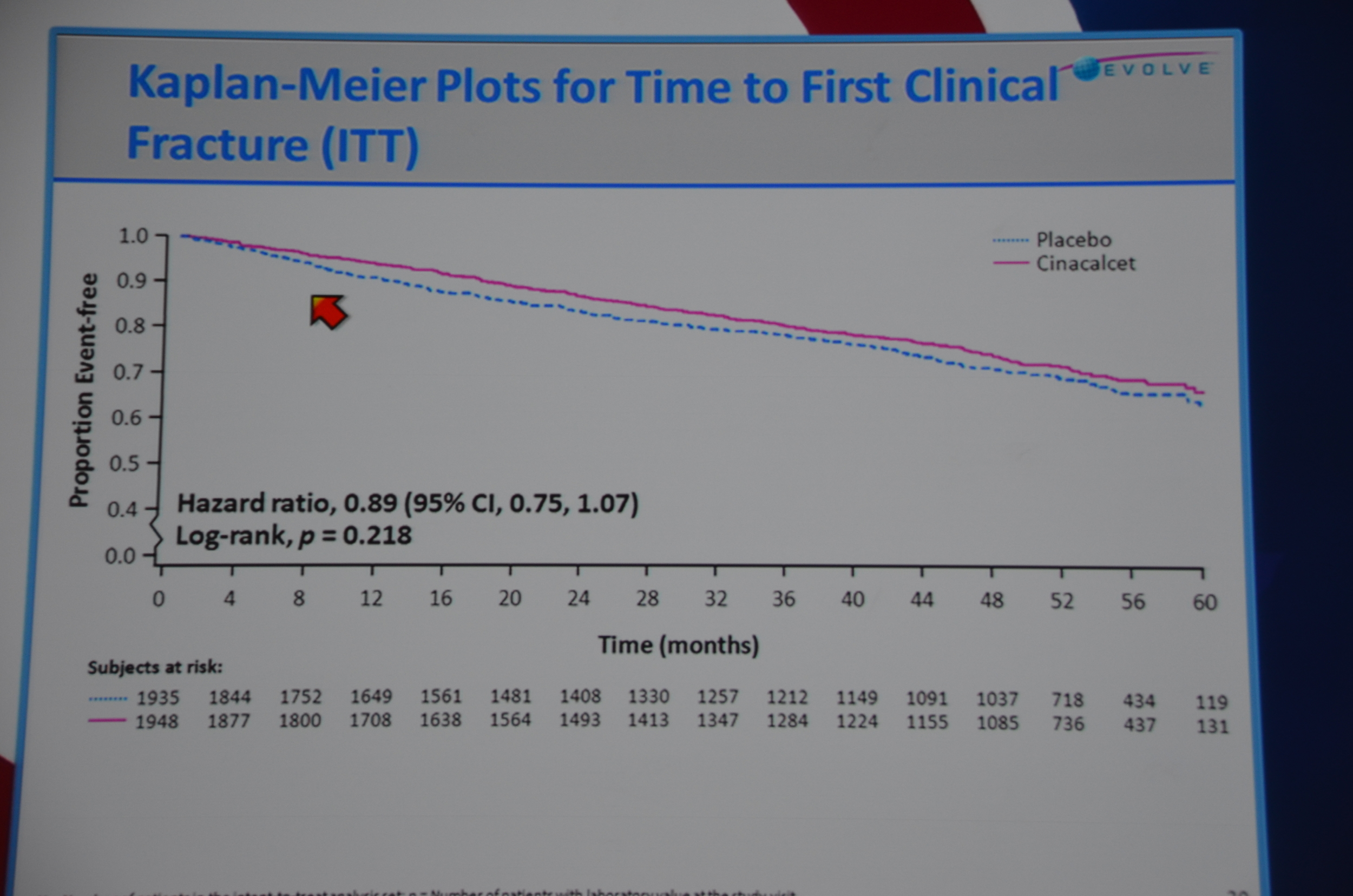
Secondary endpoints included: fracture, parathyroidectomy, CV death, stroke, and all the individual components of the primary end-point.
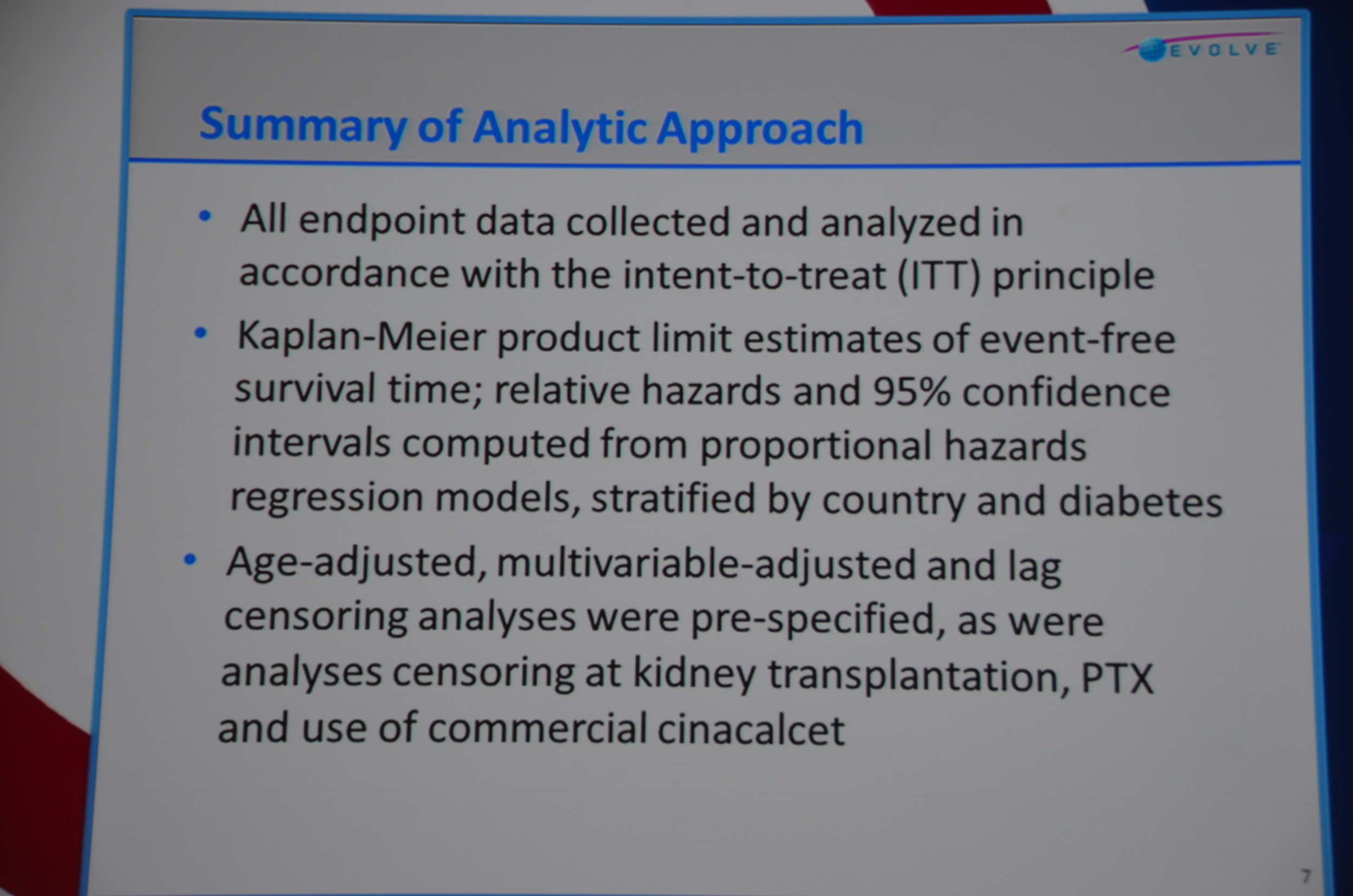
The next slide was a summary of the statistical approach. Note: when the authors present the statistical approach in a 12 minute presentation, buckle your seatbelt for a negative trial that, if you squint your eyes just the right way, looks positive.
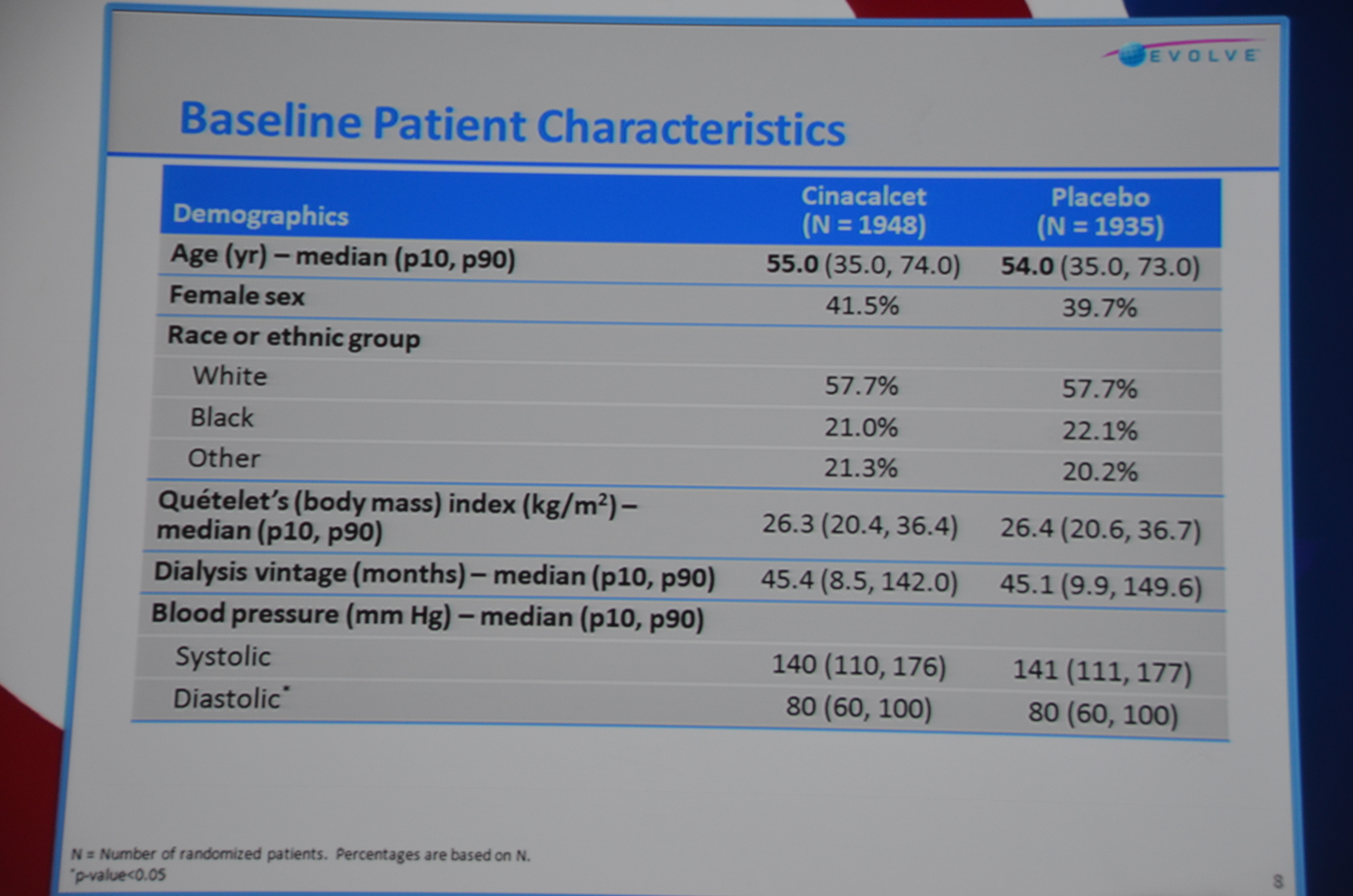
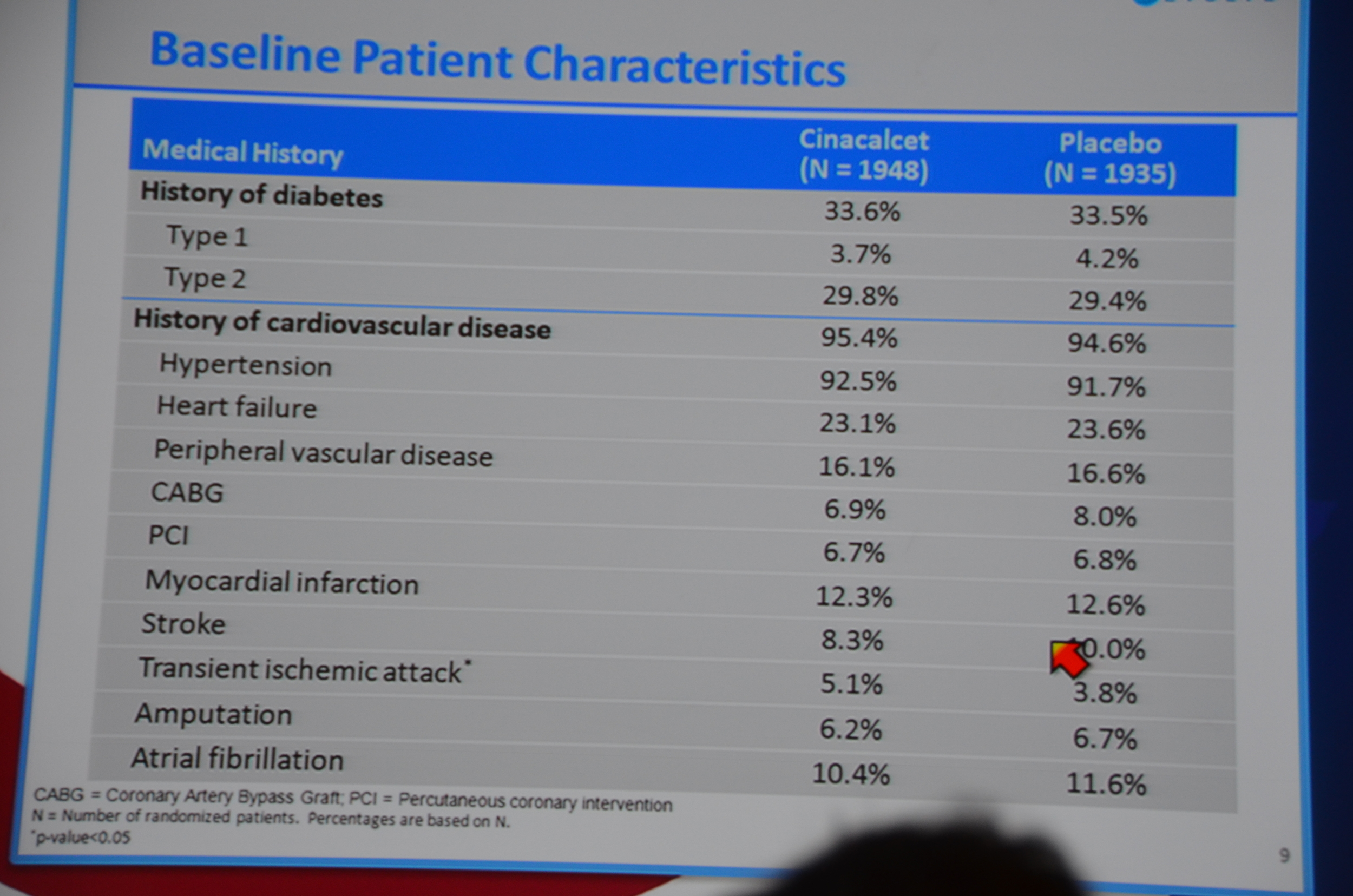
Baseline characteristics looked well balanced; however, the cinacalcet group was older, 55 versus 54. Note: at a subsequent investigator meeting, the study designers shared that there was only an 8% chance that a 4,000 person study would find that large of a difference in ages. If they had to do it all over again they would put some age restrictions in so the potential age range would be smaller, decreasing the chance of the study being unbalanced. Foreshadowing: this will become more important as the story unfolds.
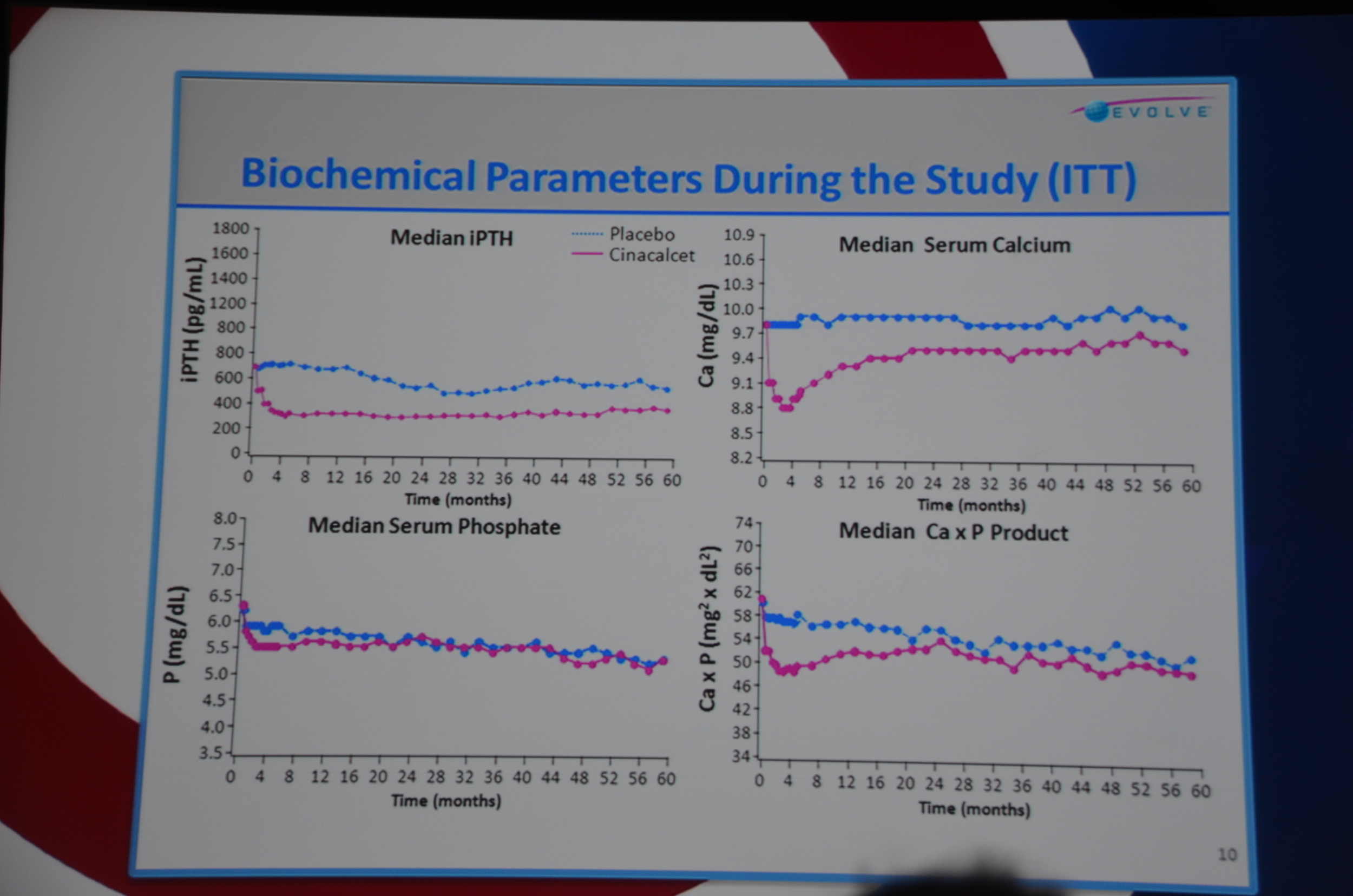
The first result they show are the biochemical parameters.
- Decrease in PTH (about 200 pg/ml lower)
- Decrease in calcium (about 0.4 mg/dl lower)
- Decrease in Ca x P product
- No decrease in phosphorous. Phosphorous has been about 10% lower with cinacalcet other trials. This did not materialize here.
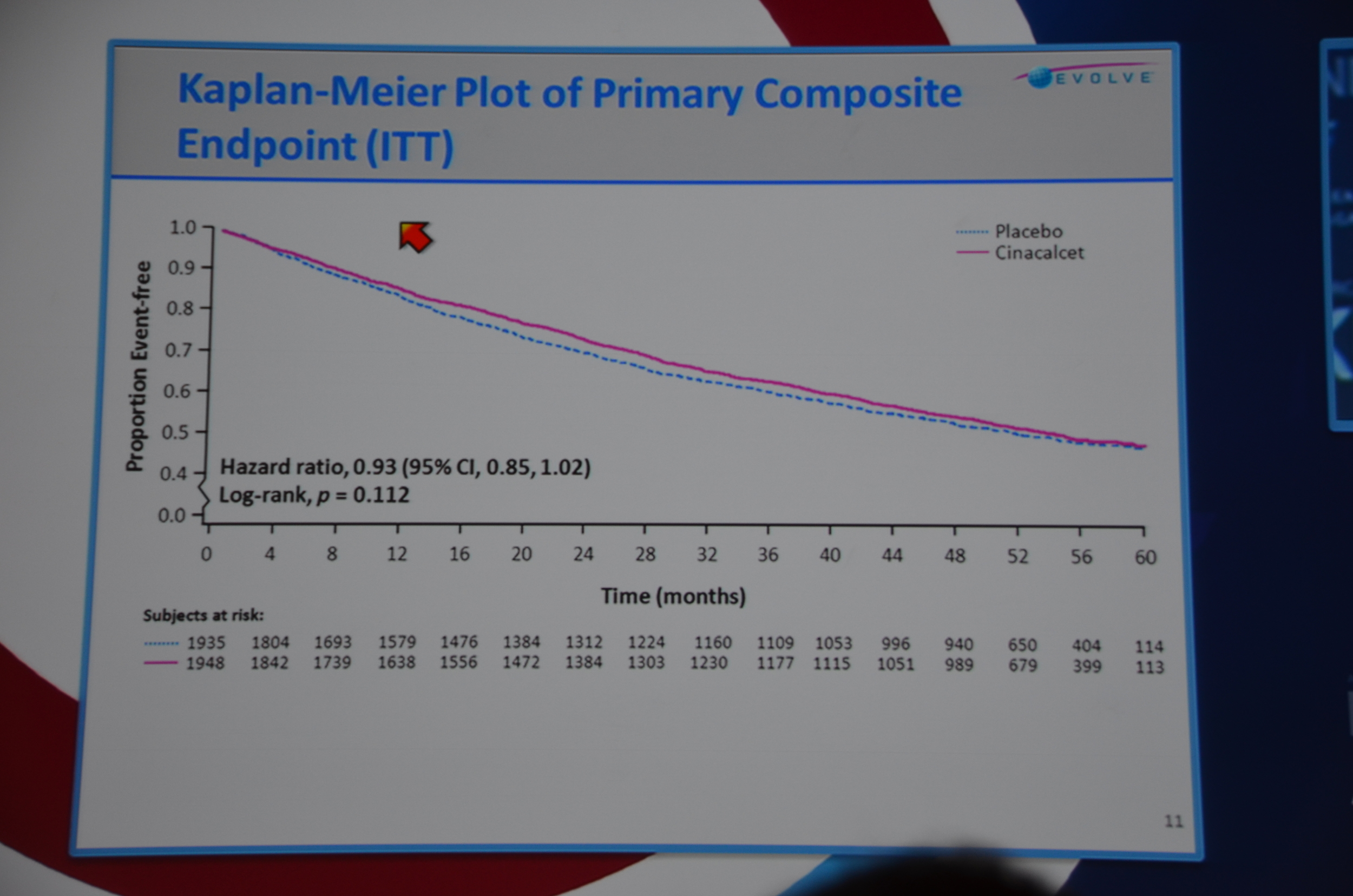
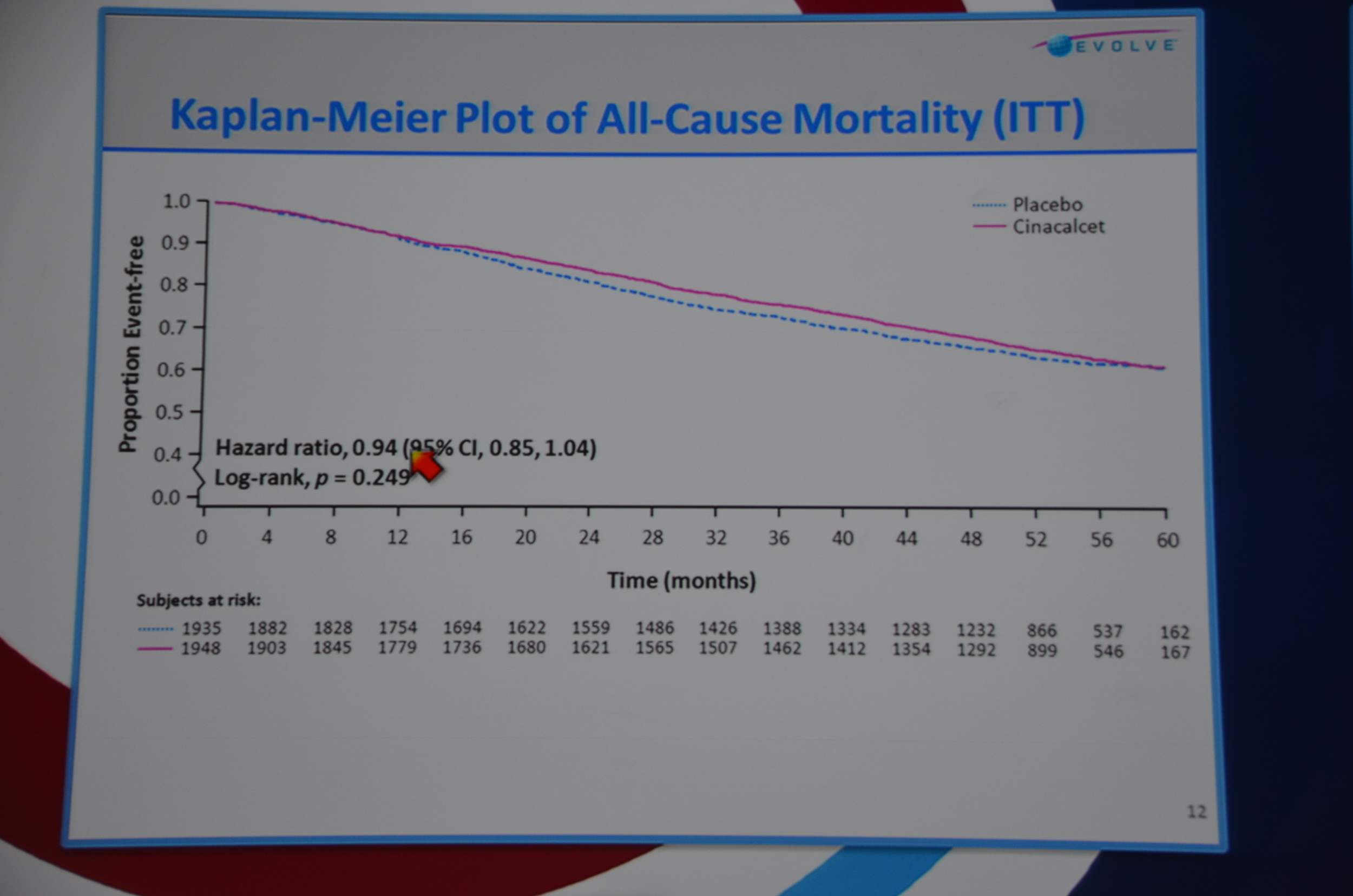
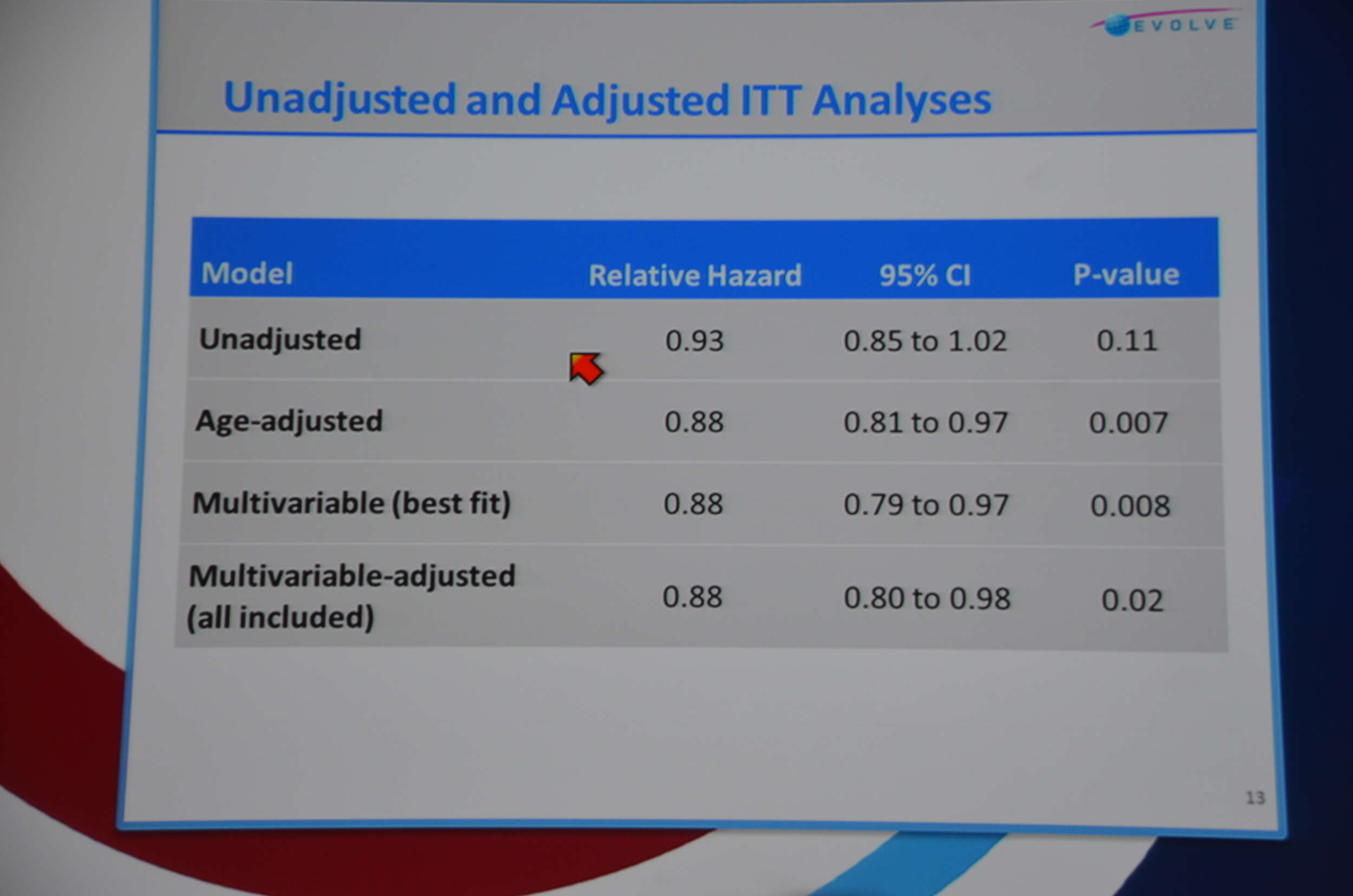
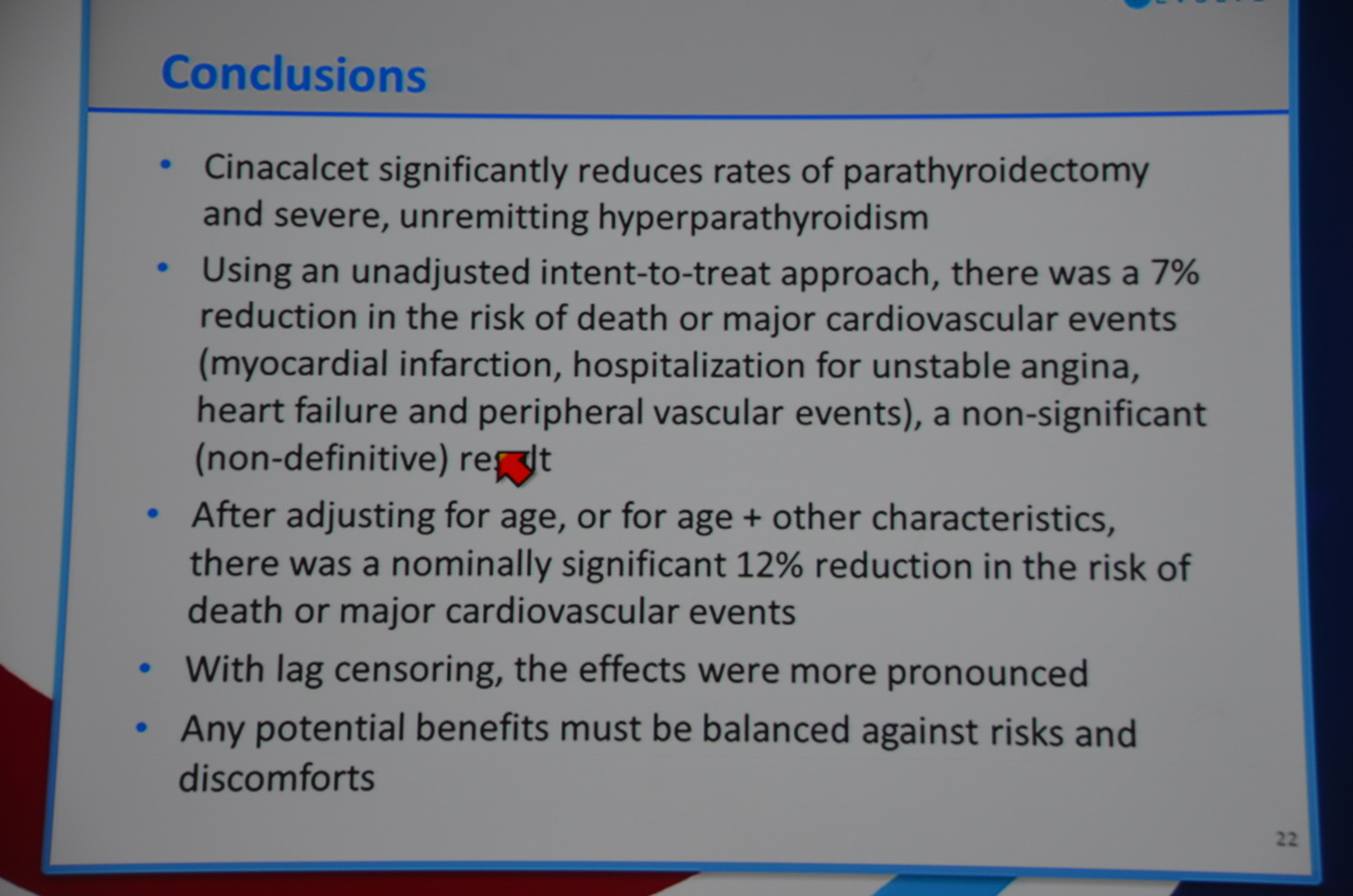
Next slide primary endpoint: HR 0.93 P=0.112. Not significant. All-cause mortality HR 0.94 P=0.249. Not significant.
They then re-ran the data to balance the ages. Age adjusted relative hazard: 0.88 P=0.007. Multivariable (best fit) HR 0.88 P=0.008 and multivariable-adjusted (all included) 0.88 P=0.02. Wow. EVOLVE runs into an unbalanced age distribution and once the data is age adjusted they reach significance. Brutal bad luck. In terms of statistical-juke-and-jive, this is pretty mild. All they had to do was adjust the data for age and, boom, significant P-value.
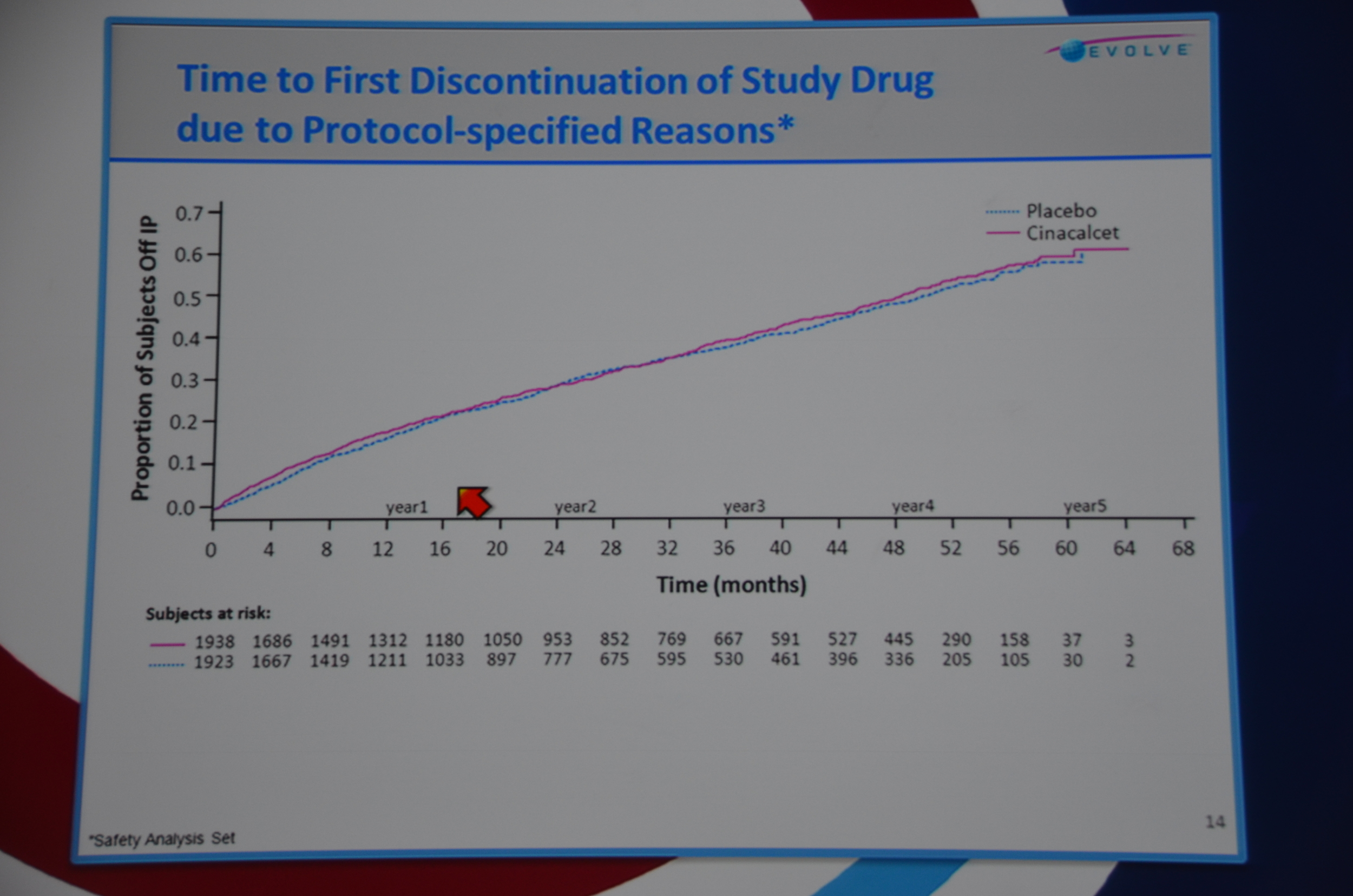
Then they looked into why people stopped the drug. Time to first discontinuation of study drug for protocol-specified reason (transplant, parathyroidectomy, etc.) was balanced across both groups but for non-protocol reasons drop out was quicker and more common for placebo. This is unusual; usually side effects cause patients to drop out of the experimental group faster than the placebo group. Presumably this was due to patients dropping out of placebo due to uncontrolled PTH and doctors switching them to commercial cinacalcet. Problems only seen when doing an RCT of an approved drug.
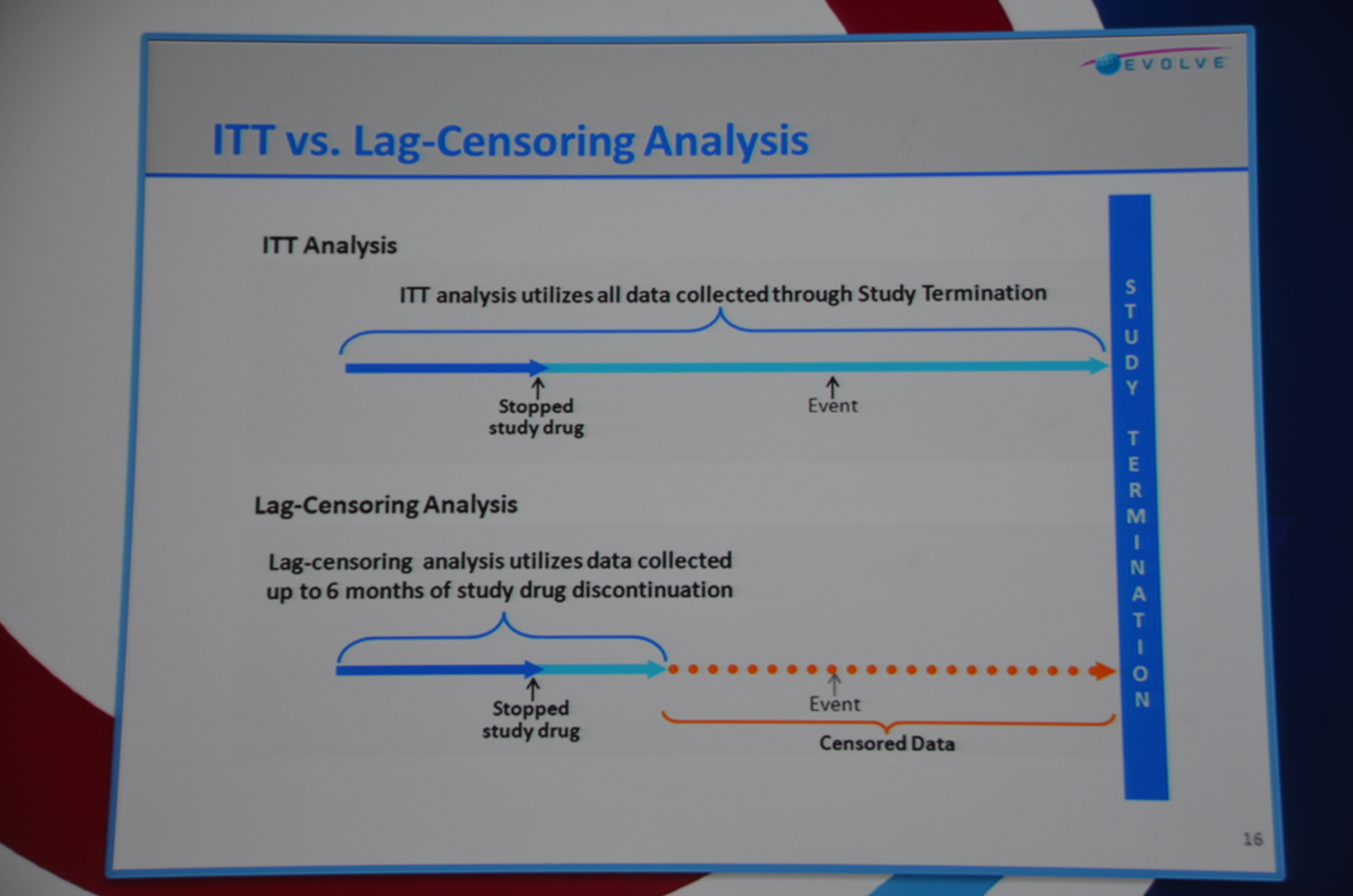
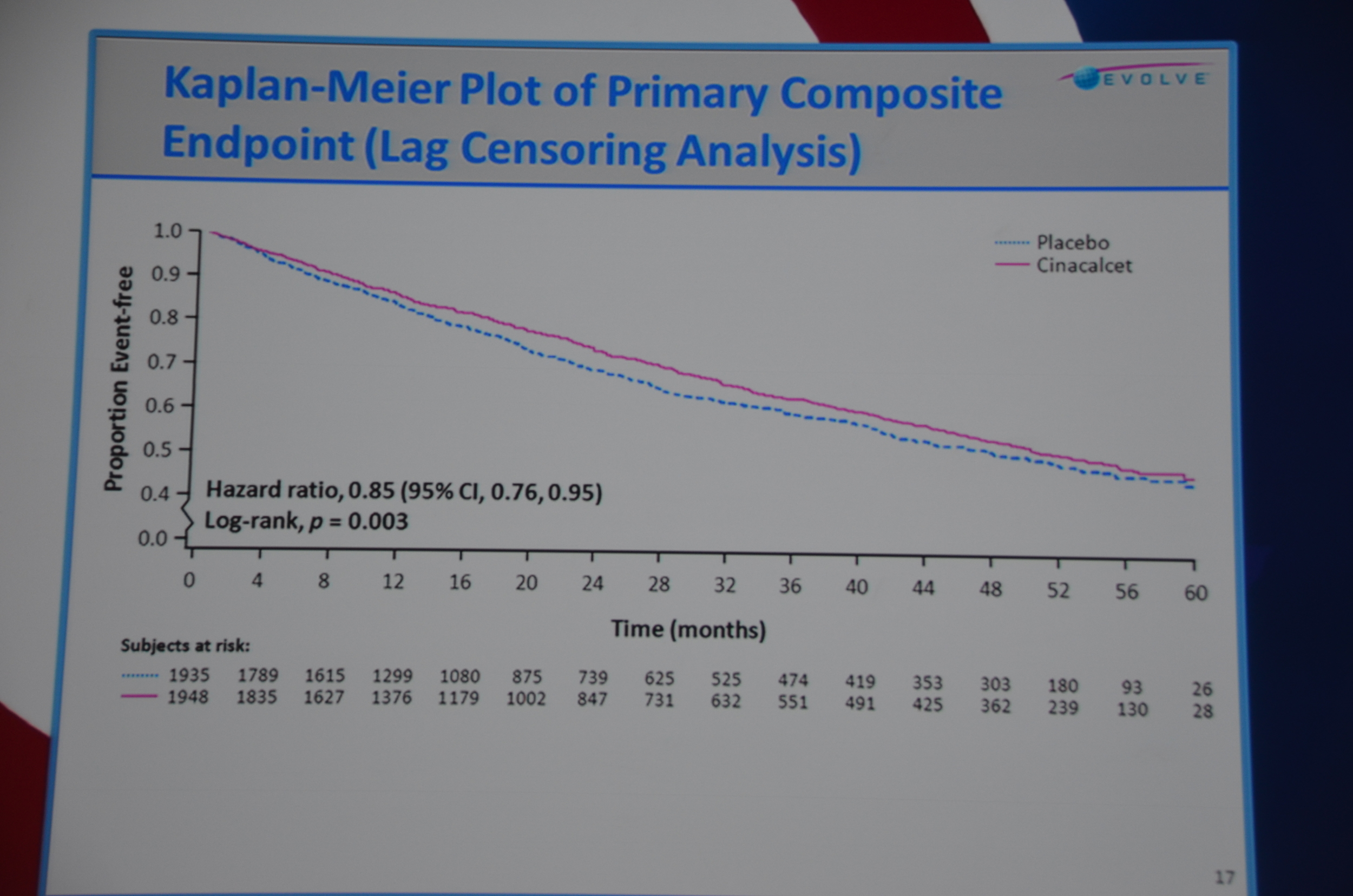
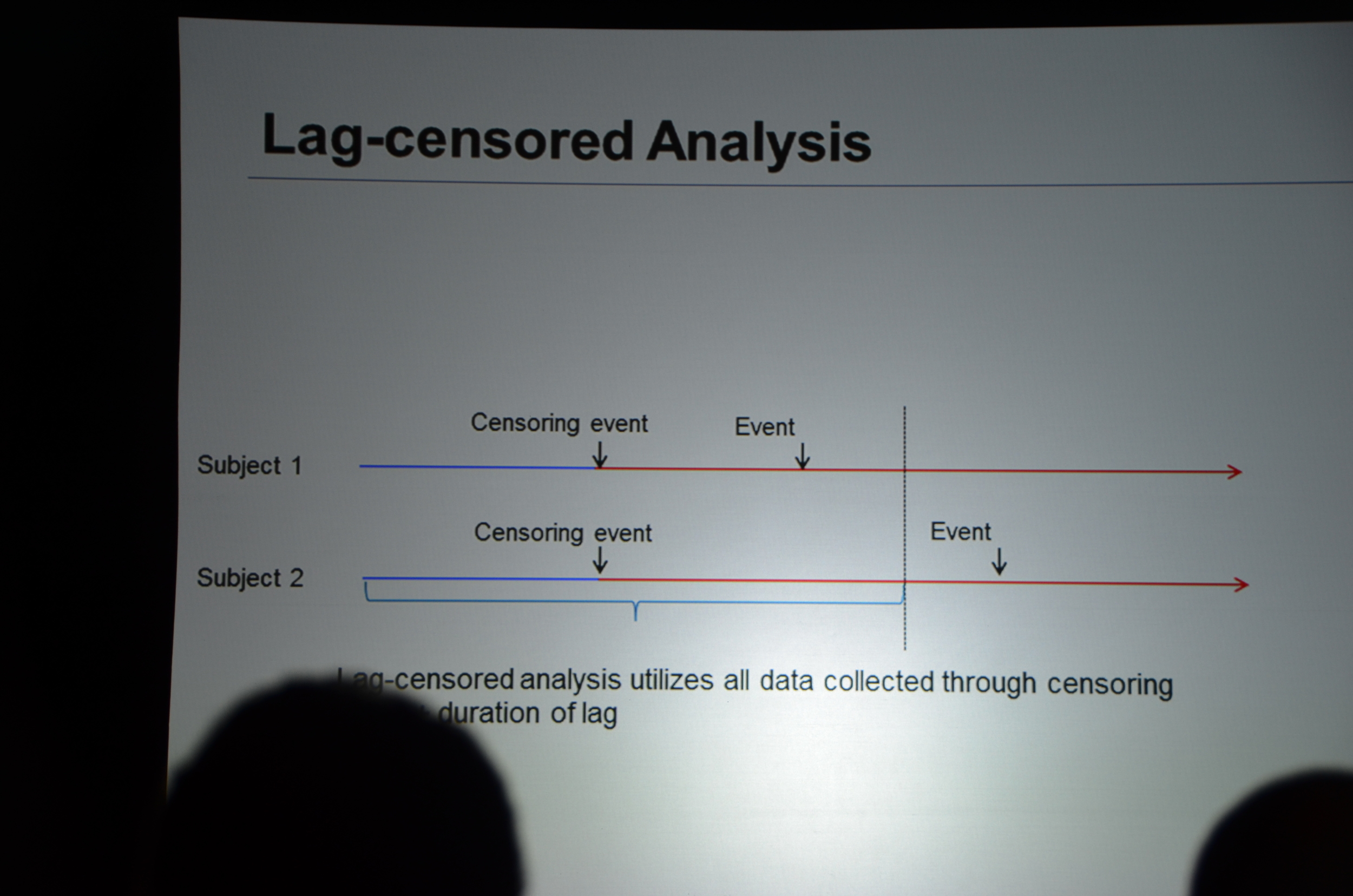
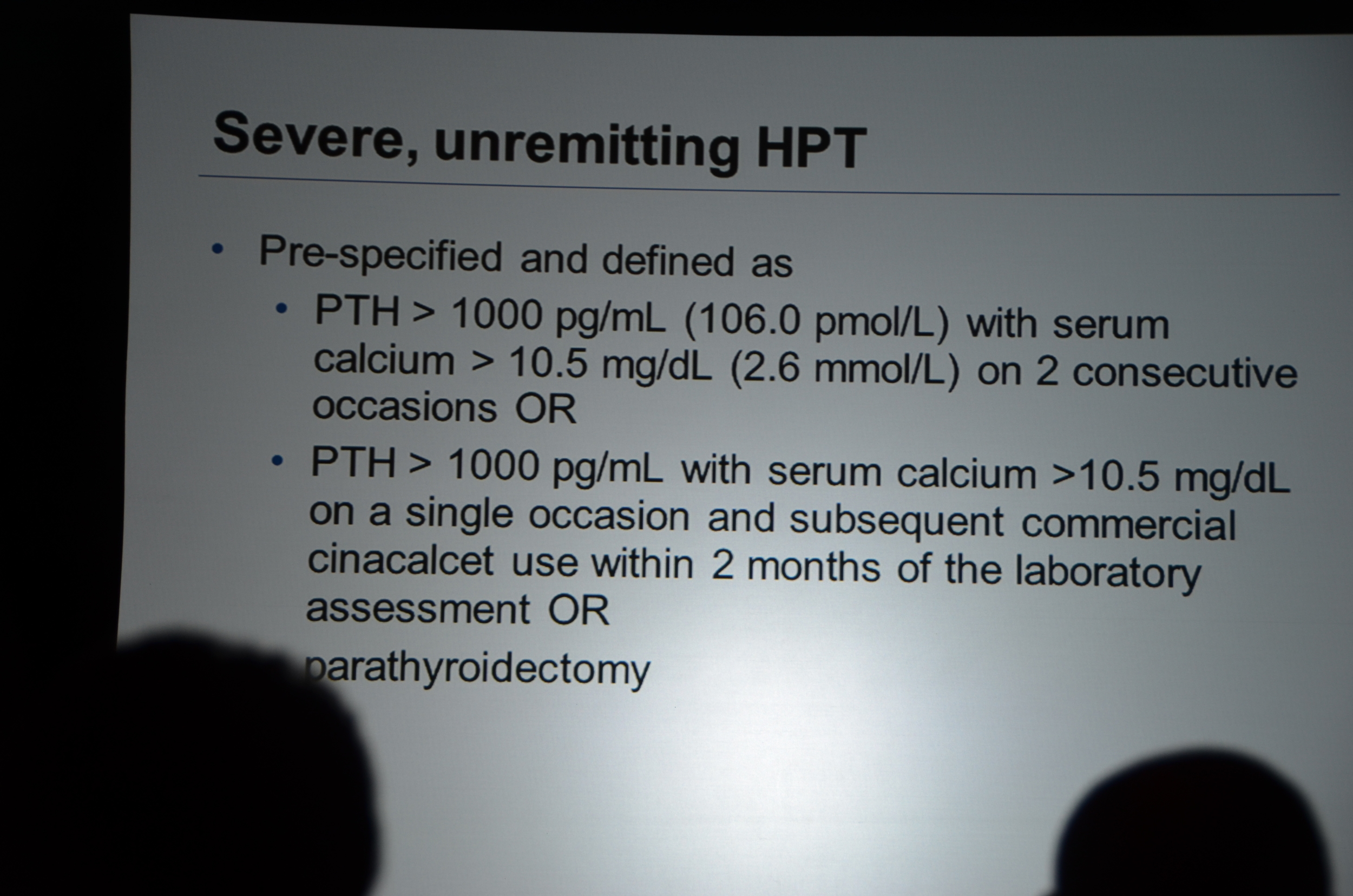
Dr. Chertow then discussed lag-censored analysis. This is a modified approach to intension-to-treat analysis (ITT). ITT is the most rigorous approach but the designers of EVOLVE pre-specified a lag-censored approach. The idea here is that if a patient stops taking study drug, at some point in the future the effect of the drug could not explain an outcome. For example if a patient took the drug for a week, it is hard to accept that a parathyroidectomy 5 years later was due to the drug. The authors pre-specified a lag of 6 months. This means that any outcomes that occurred more than six-months after the last pill was consumed were ignored. The lag-censored analysis only censored people who stopped the drug for protocol specified reasons (transplant or parathyroidectomy were mentioned, there may be others). They found a HR for the primary outcome of 0.85 P=0.003. They found a HR for mortality of 0.83 P=0.009.
On to bone-mineral outcomes. Time to parathyroidectomy was longer with cinacalcet, HR 0.44 P<0.001. No difference in fracture risk.
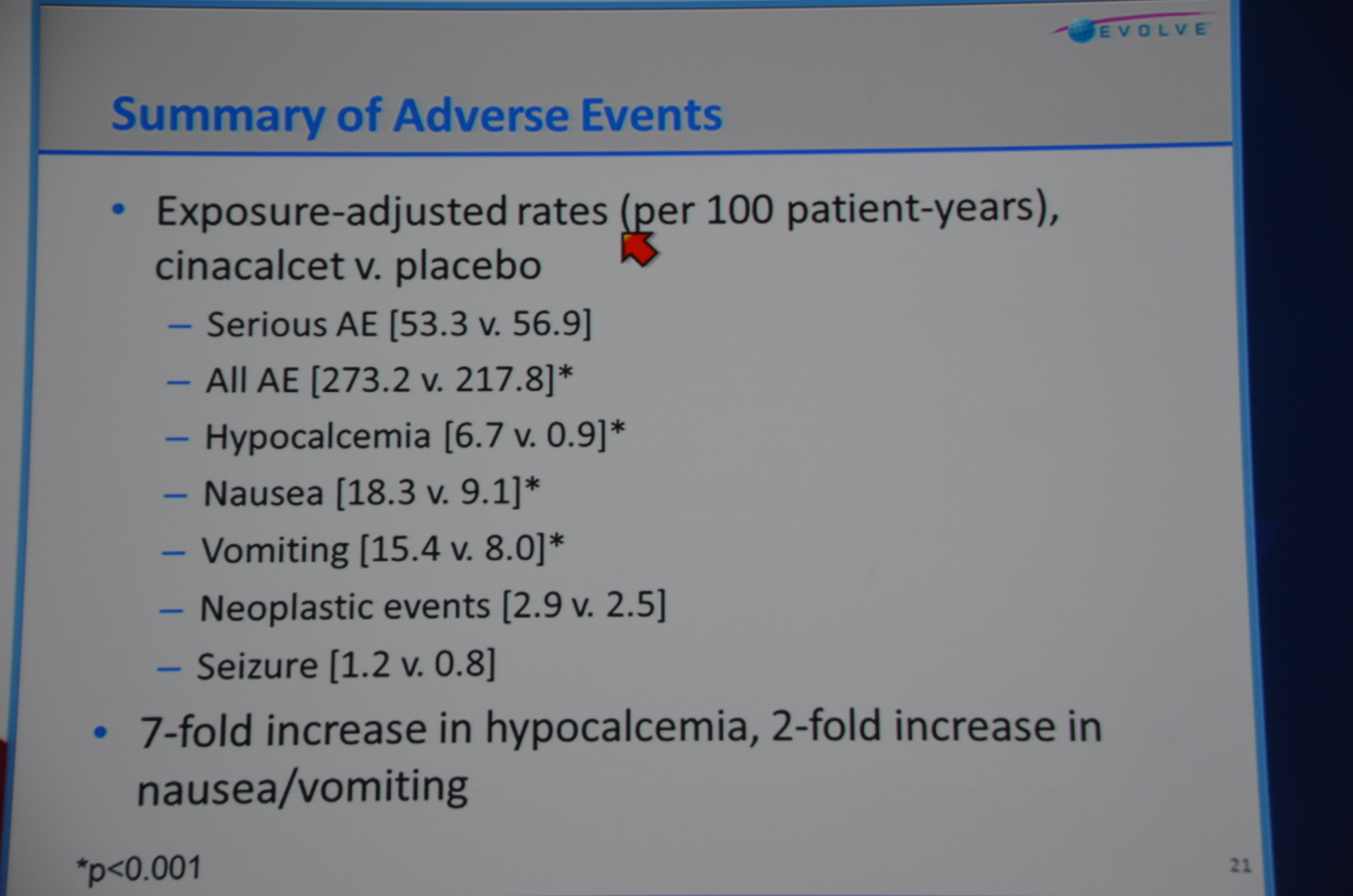
Adverse events: there were a lot. 273 per 100 patient years. Wow. Serious adverse events were also found 53.3 per 100 patient years. The only ones that were significantly (P<0.001) more common with cinacalcet were unsurprisingly: All AE (273 vs 217), hypocalcemia (6.7 vs 0.9), nausea (18.3 vs 9.1), vomiting (15.4 vs 2.5).
Final thoughts, the drug failed its primary endpoint and it looks like one of the main reasons is they ran into an unbalanced distribution of ages. This was a larger, long and in terms of double blinding, unprecedented study in dialysis, there were a hundred reasons it could have failed, the fact that it was something as simple as unbalanced age distribution is kind of tragic.
Dr. Chertow mentions that EVOLVE is going to be published online in the New England Journal of Medicine and described the discussions with the editors as being “spirited” on more than one occasion. Sounds like there were some fireworks pounding the final manuscript together.
Post written by Dr. Joel Topf, eAJKD Advisory Board Member, and edited by Dr. Vinay Nair, eAKD Advisory Board member.
Transcript of my live Tweet Stream
eAJKD Next up Evolve and Glenn Chertow! #KidneyWk12
eAJKD Sensipar in ESRD end-point total mortality. Very exciting, largest dialysis study ever. #KidneyWk12
eAJKD To be dropped in NEJM with 67 page appendix. #KidneyWk12
eAJKD He describes the experience with NEJM as "Spirited" sounds a bit stickly #KidneyWk12
eAJKD RCT placebo controlled double blinded. Other therapies for PTH /mineral metabolism not blinded or restricted. #KidneyWk12
eAJKD Event driven trial. We run it until enough people die or reach CV endpoint. #KidneyWk12
eAJKD Groups were well balanced #KidneyWk12
eAJKD No difference in phos, decrease PTH and Calcium with cinacalcet. PTH looks 200 lower and Ca 0.7 lower. #KidneyWk12
eAJKD Primary end point look identical, 7% rediuction with cinacalcet, P=0.112 NS #KidneyWk12
eAJKD 6% reduction in mortality P=0.24 #KidneyWk12
eAJKD Age adjusted ITT data is sig HR 0.88 P=0.007 #KidneyWk12
eAJKD Lag censoring analysis versus ITT, this was a pre-specified analysis. This tries to look at lasting effect after D/C drug #KidneyWk12
eAJKD This was for people who stopped drug for transplant or parathyroidectomy. HR 0.83 P=0.009 #KidneyWk12
eAJKD Reduction in time to first parathyroidectomy, and time to unremitting hyperparathyroidism #KidneyWk12
eAJKD No reduction in fractures #KidneyWk12
eAJKD No suprises in adverse events, we know this already approved drug. #KidneyWk12
eAJKD I was an investigator in EVOLVE so I may be biased but I'm pleasantly surprised. The most positive dialysis study ever? #KidneyWk12
Here's my editorial from PBFluids
Do we need to EVOLVE our views on EBM in dialysis
I have posted on the release of the EVOLVE data at Kidney Week 2012 at the eAJKD blog.
This is a big deal and when I read the @NEJM tweet it makes me mad.
Cinacalcet doesn’t lower death or major CV event risks in patients undergoing dialysis.nej.md/U4yeSv #kidneywk12
— NEJM (@NEJM) November 3, 2012
That tweet provides only the first sentence in what should be an important and longer discussion of the findings of EVOLVE. To completely discount EVOLVE as @fish2phil and @Nephroboy do...
@kidney_boy these are statistical machinations attempting to squeeze a diamond out of coal.
— Homer W. Smith (@fish2phil) November 4, 2012
It's cack. "@kidney_boy: My first take on EVOLVE: biggest, longest, RCT ever undertaken in dialysis population ajkdblog.org/2012/11/04/kid…"
— Nephroboy (@nephroboy) November 4, 2012
...leaves the nephrologist in a bit of a bind. If we ignore studies that don't meet their primary endpoint, what are we left with? Here is a list of ALL the randomized controlled trials in dialysis that use hard outcomes. This list ignores any study where the end-point is an improvement in a biochemical parameter, echographic finding, or vital sign:
- NCDS from 1981: Positive. We learned that small molecule clearance is better than large molecule clearance
- Normalization of hematocrit: Negative. No advantage and nearly significant disadvantage to normalizing the hemoglobin
- HEMO: Negative. Definitively negative trial showing that small molecule clearance can not get any better with thrice weekly hemodialysis
- ADAMEX Negative. Peritoneal dialysis version of HEMO, same result
- AURORA Negative. Statin trial with rosuvastatin
- 4D Negative. Statin trial with atorvastatin
- SHARP Positive, kind of. Statin plus ezetimibe was able to reduce CV endpoints however they lumped dialysis and CKD patients together. The dialysis patients alone were not powered to find a difference and they did not. I think most nephrologists, in the face of 4D and AURORA are pretty skeptical of this data approach.
- DCOR Negative. Trial of sevelamer versus calcium containing binders.
- IDEAL Negative. Trial of early versus late initiation of dialysis. No advantage for early start. Some may argue that this is a CKD rather than dialysis study.
- EVOLVE. Negative.
Is that it? I can't think of any others. Nephrology operates in an evidence desert. (Hat tip Dr. Dale)
I’m a nephrologist because I’m comfortable operating in a low evidence environment.#FaithBasedSpecialty
— Joel Topf (@kidney_boy) November 3, 2012
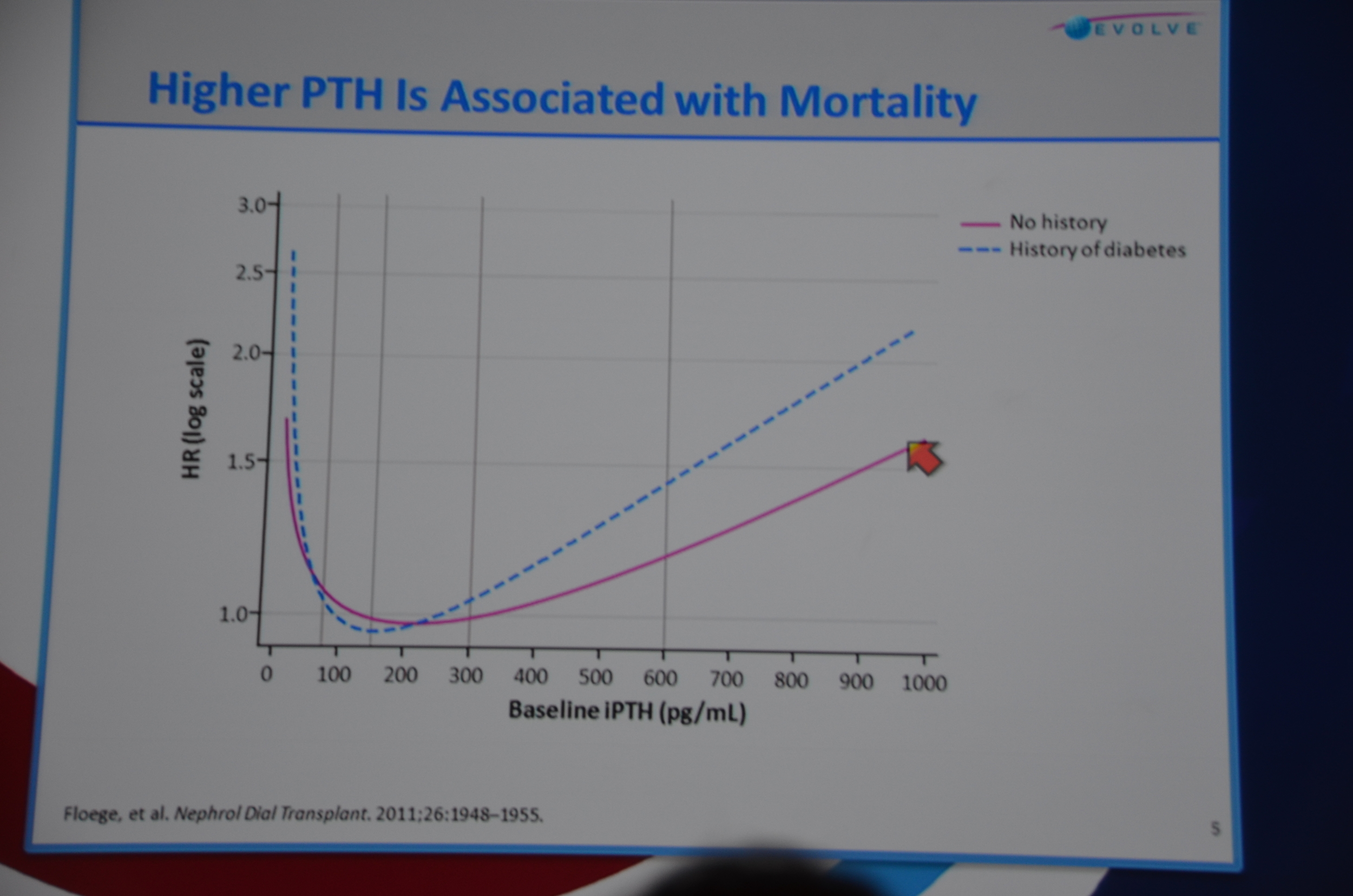
In a few weeks I will start going through the November labs for dozens of patients who trust me to give them the best dialysis care. Some of them will have a PTH over 600 and a calcium over 8.4. What will I do? If I am relying on randomized controlled data I will probably just sit in a corner and cry. I could rely on Block's, Floege's andKalantar-Zedah's data that ties increased PTH with increased risk of death. All of that data is, of course, controlled for age the sin for which Homer Smith is pounding the EVOLVE crew for:
@kidney_boy …not only adjusting for age, but may really be adjusting for important unmeasured covariates that correlate with age.
— Homer W. Smith (@fish2phil) November 5, 2012
To see what happens when you lower the PTH we need to look at the data from the mortality studies done with paricalcitol (Zemplar) and doxercalciferol (Hecterol).*
* The reason there is no link to those studies is that those studies don't exist. Thanks Abbott. Thanks Genzyme. I have heard rumors of a Zemplar trial conducted in the 90's but the data was never published and the study buried in the bad old days before trials.gov.
So what is the EBM dedicated nephrologist to do?
I bet if the skeptical nephrologist went to his dialysis patients and explaned to them:
USRDS 2012 Annual Data ReportUSRDS 2012 Annual Data Report
- Dialysis patients have a 3 year survival of 50%.
- There is a drug that is already approved (i.e. not experimental) that recently has been shown to have an 89% chance of being able to reduce mortality by 17%
That every one of his patients would beg for the drug.
So if you want to throw EVOLVE away because it didn't reach statistical significance what are you going to do? The questions that EVOLVE attempted to answer do not go away because the study was negative.Of course if you accept the data that elevated PTH is bad, none of the observational data provides any guidance on what happens when you lower PTH.Final thoughts: The first time I heard about intention to treat was during the HIV epidemic. Some of drugs were so toxic, especially in the early days of HAART when patients were taking handfuls of pills, that significant number of patients weren't able to tolerate them. I remember an ID doc expressing frustration that he needed to know if the drug would save the lives of the patients he had that could tolerate the drug. If they can't tolerate it, he was going to stop the drug, but if they could tolerate it he needed to know if it slowed the virus. In the middle of the epidemic, there is no room for statistical purity.
Like that ID doctor, I want to know if the drug will work if the patient actually swallows the pill, and the answer to that is a definitive "Yes." Hazard ratio for death of 0.83 P=0.009.
Disclosures: I was a principle investigator in EVOLVE, that means my practice was paid to recruit, and administer a bitch of a study that lasted longer than it was supposed to. It was a lot of work, a lot of meetings, a lot of signatures, a lot of responsibility, and not so much money. My name will not be among the authors.
My name on page 53 on the supplement
My name on page 53 of the supplement
“think of time censored analysis as a stop between ITT and as treated. It is a compromise position, more rigorous than as treated, and less rigorous than intention to treat.”
ALTITUDE
ALTITUDE
PURPOSE
Altitude was designed to see if adding aliskiren to patients with diabetes resulted in a decrease in cardiovascular or renal end-points. We had already seen Alikiren reduce proteinuria in patients with diabetic nephropathy so based on the assumption that reducing proteinuria was a valid surrogate end-point the authors pursued the definititive data of reduced CV and renal end-points.
ENROLLMENT:
Type diabetes with one of the following risk factors
- macroalbuminuria and GFR >30
- microalbuminuria and CKD stage 3
- CKD Stage 3 and history of CVD
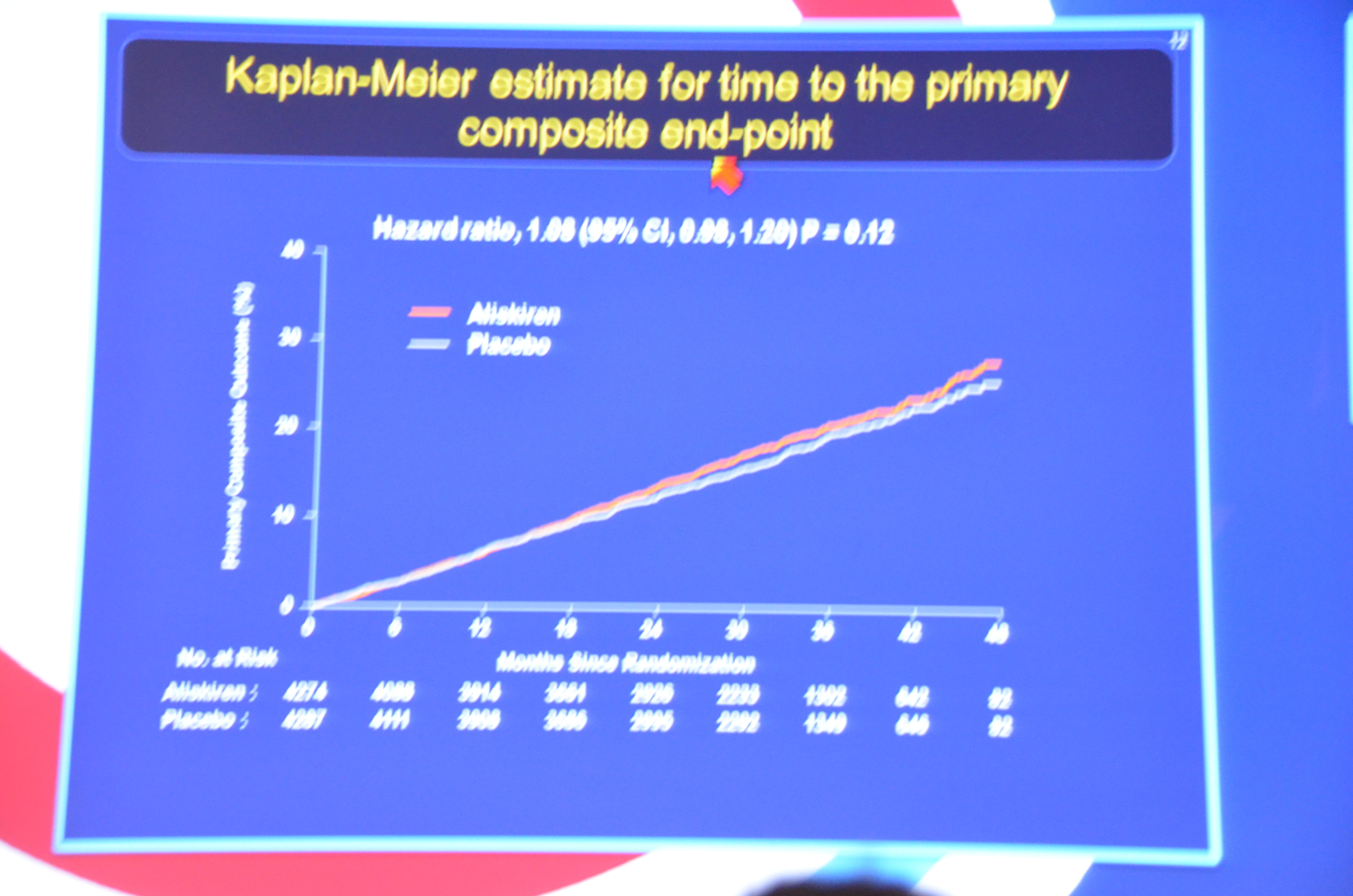
The study was stopped early due to futility.
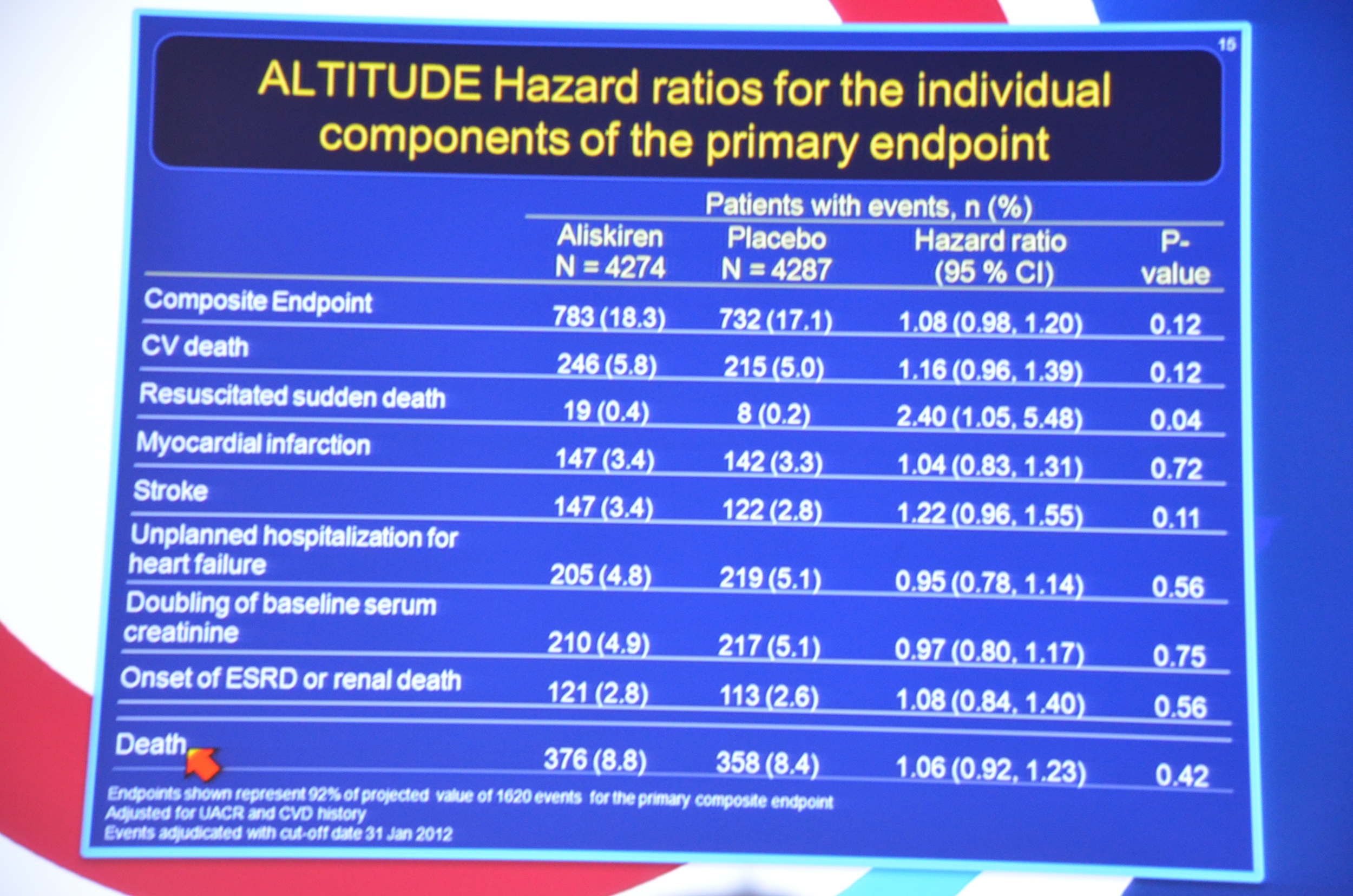
...the primary end point had occurred in 783 patients (18.3%) assigned to aliskiren as compared with 732 (17.1%) assigned to placebo (hazard ratio, 1.08; 95% confidence interval [CI], 0.98 to 1.20; P=0.12).



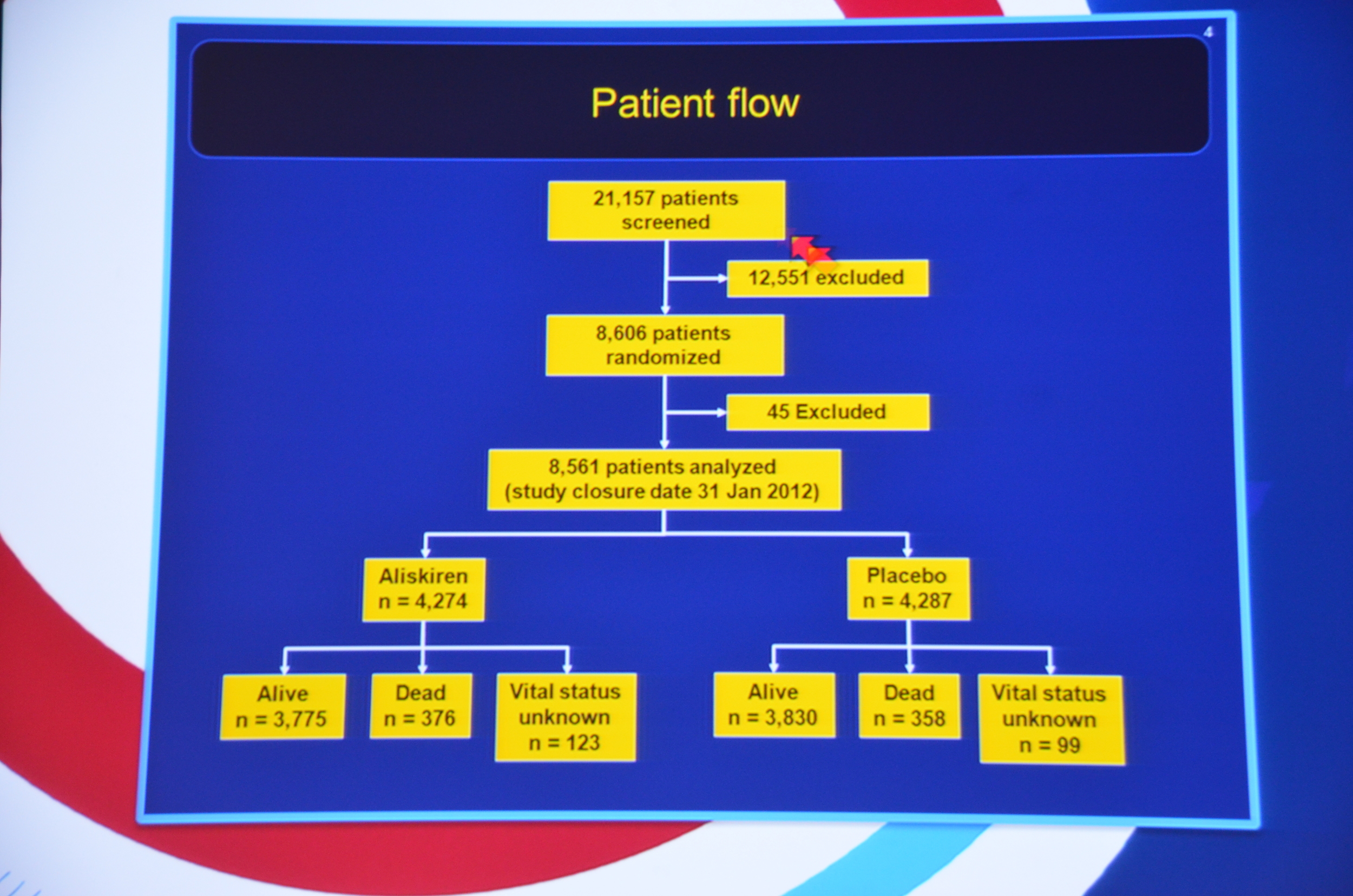
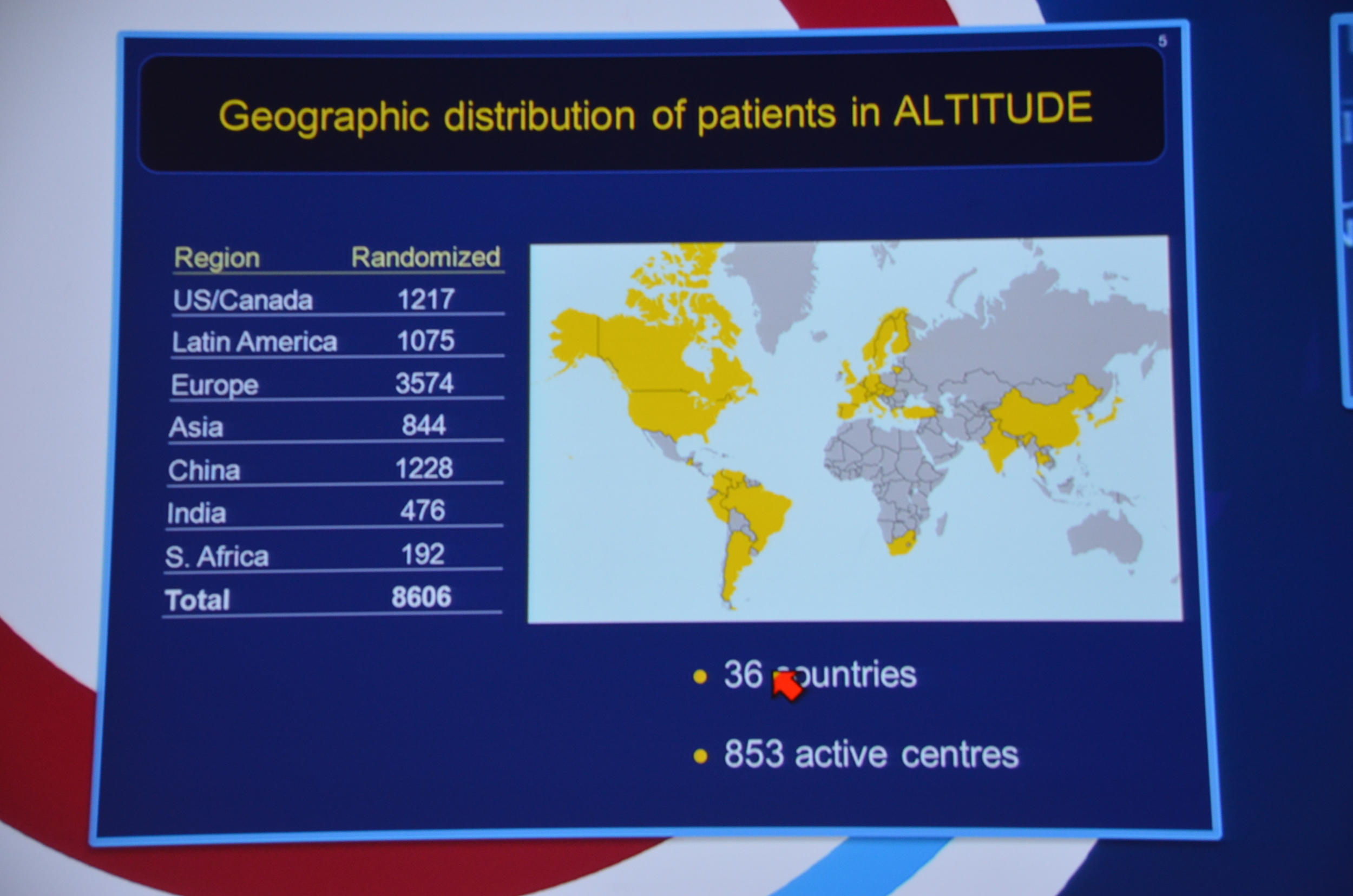



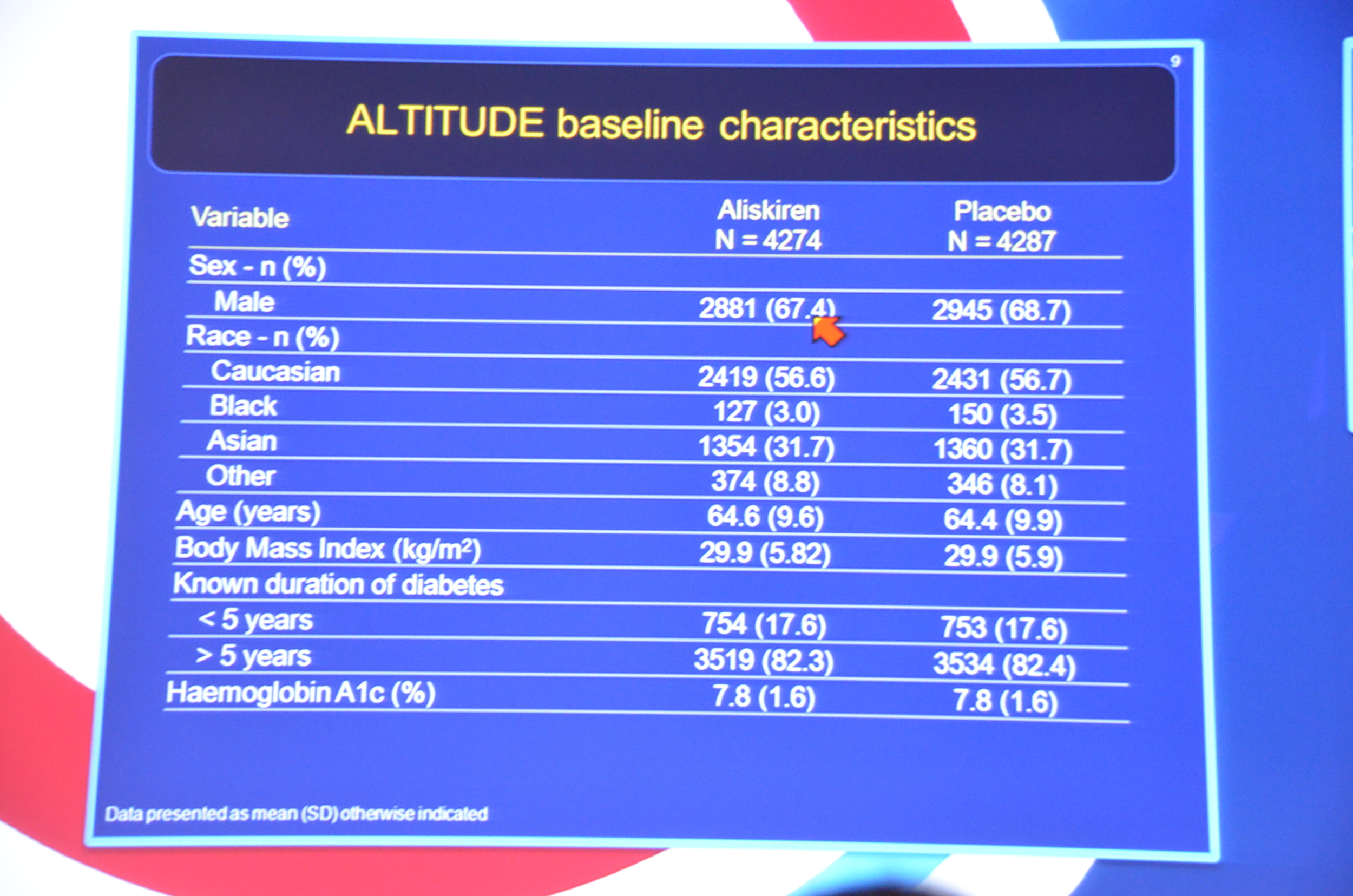
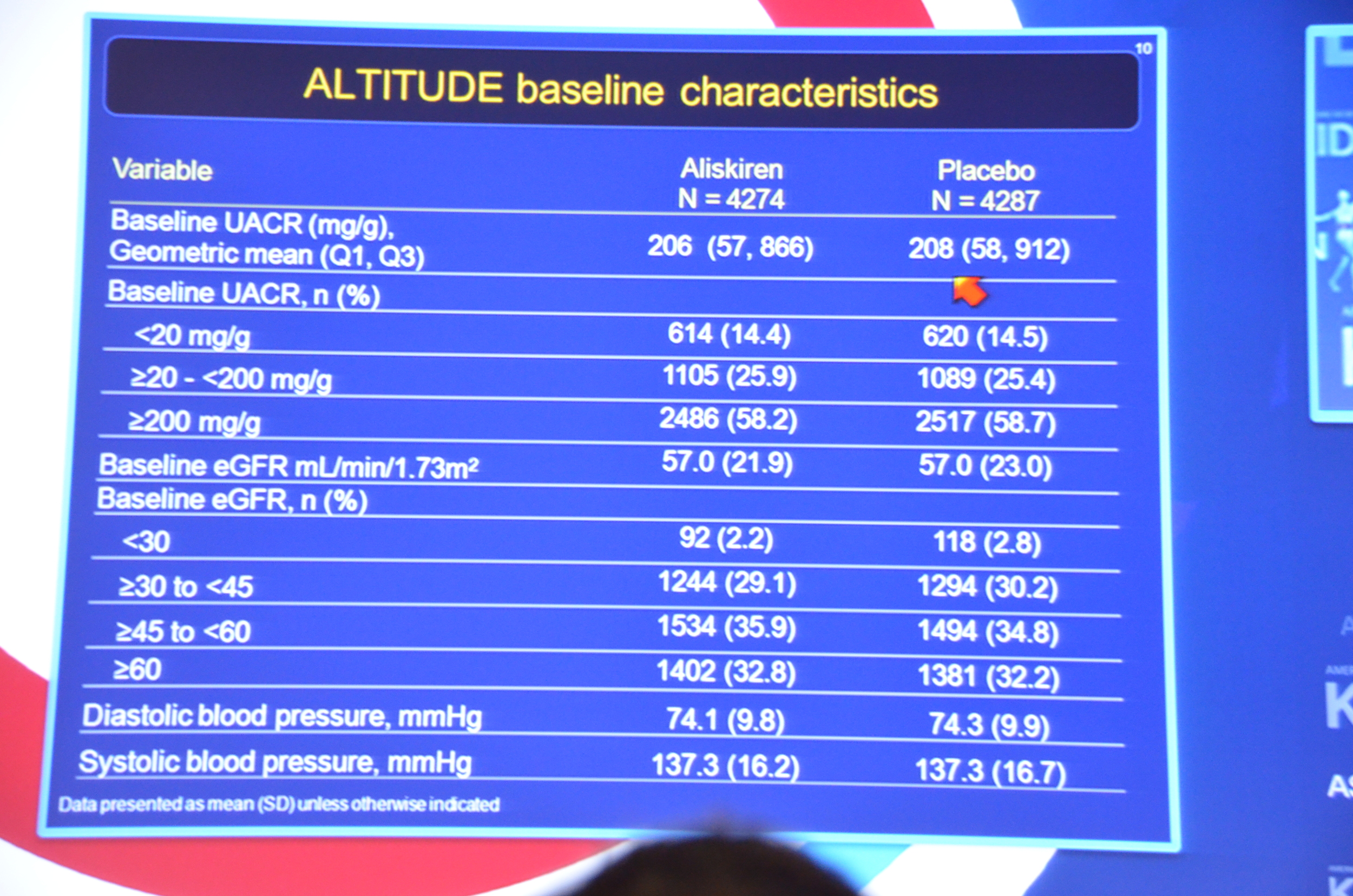
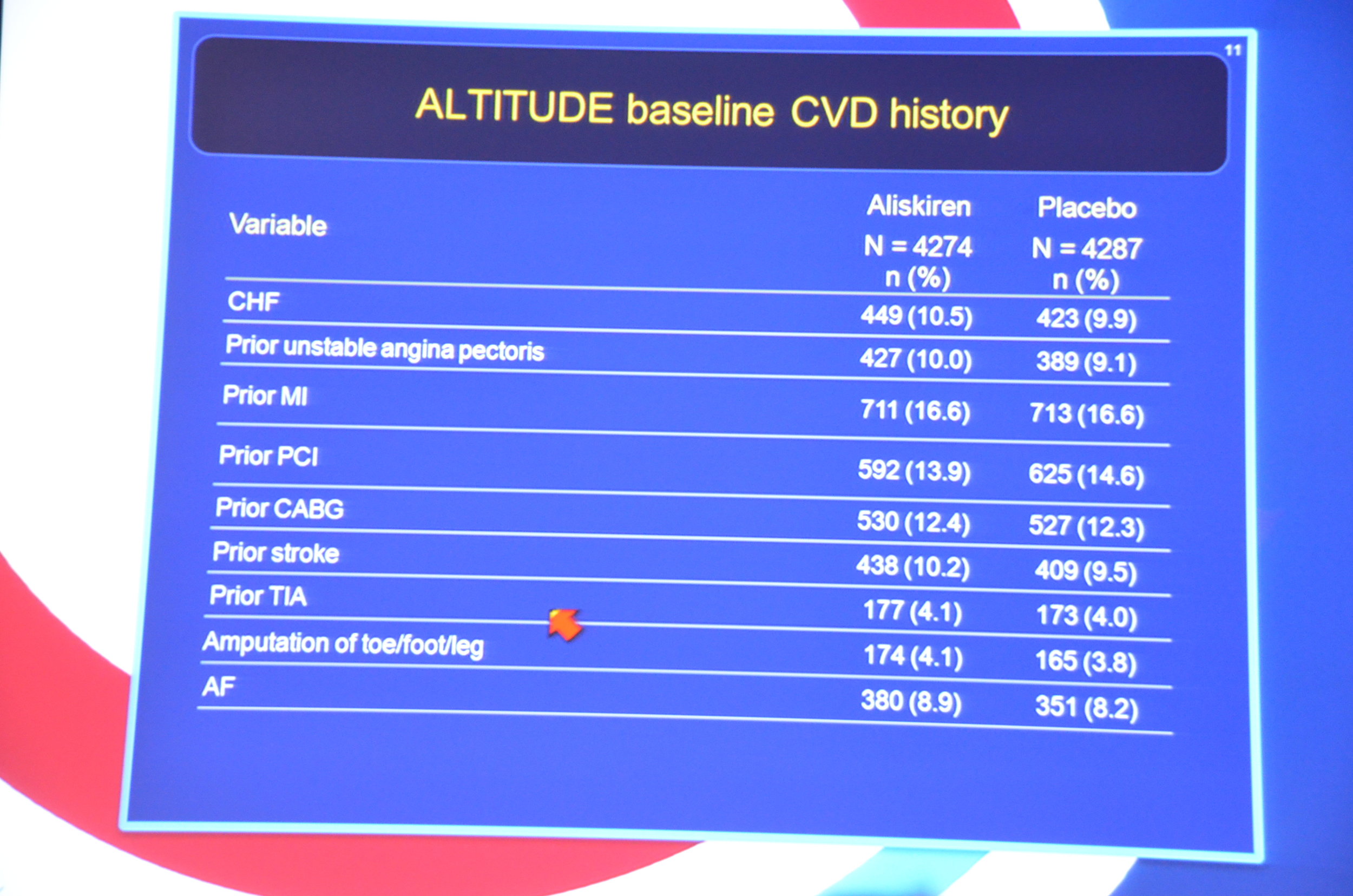


My Live Tweet Stream from the announcement
eAJKD Parving up. Talking about the failed ALTITUDE trial. #KidneyWk12
eAJKD Terminated in Dec 11 due to futility and side effects #KidneyWk12
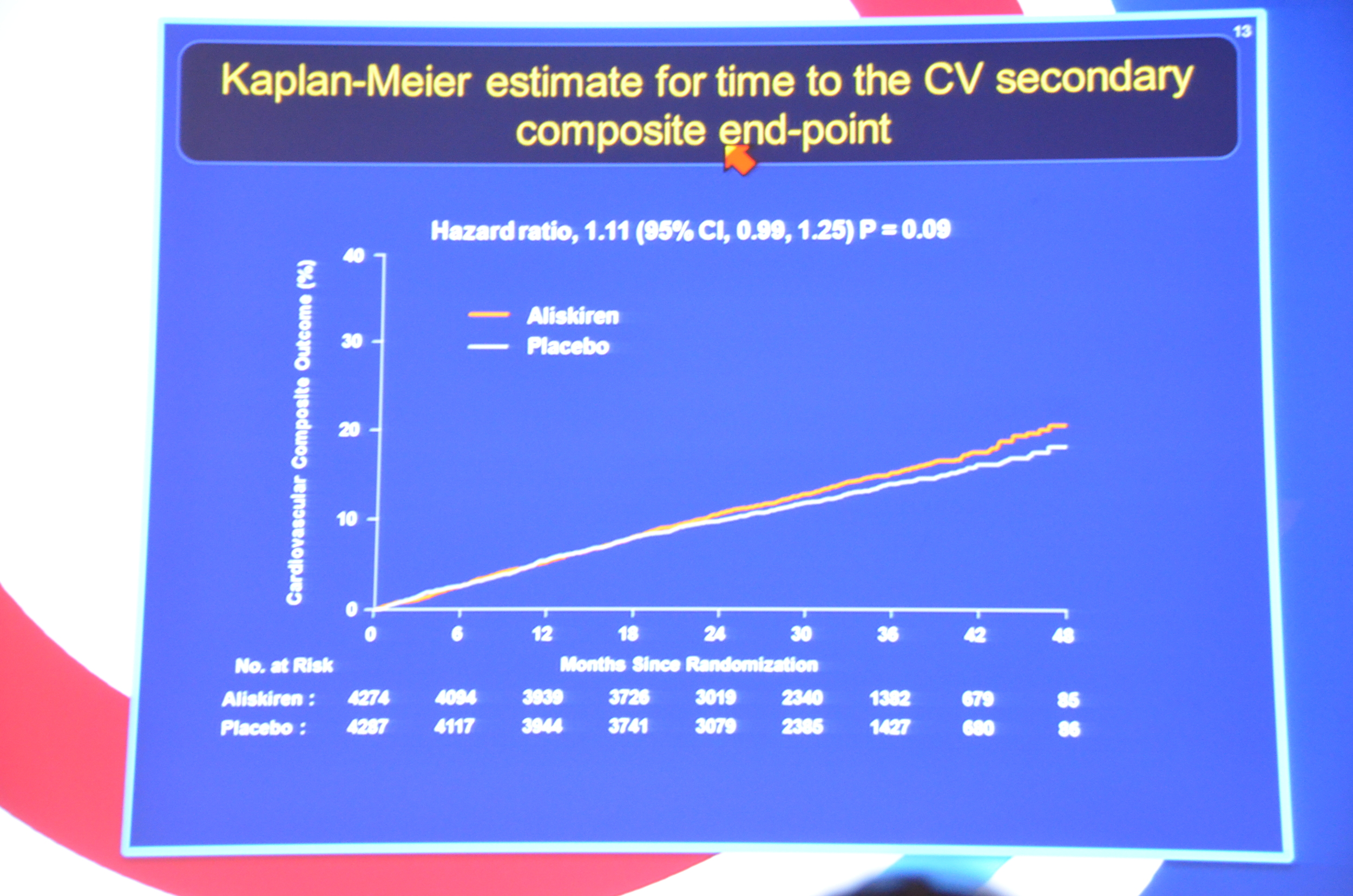
eAJKD Lots of cardiac end points in CKD-diabetes. #KidneyWk12
eAJKD 8,561 patients randomized. 3776 dead in the aliskiren358 in the placebo #KidneyWk12
eAJKD 850 centers in 36 countries #KidneyWk12
eAJKD enrollment: T2D Macroalbumin and gfr > 30 or microalbumin and GFR 30-60. Used kidney to find high risk pts. CV endpoint #KidneyWk12
eAJKD RT @KristinaMPT: So much for the NEJM embargoes (3:30 PM) RT @eAJKD About to live tweet the late breaking trials sessions at #KidneyWk12
eAJKD 82% had T2DM for > 5 yrs #KidneyWk12
eAJKD Albuminuria, average 200 mg/g Cr #KidneyWk12
eAJKD 17% with prior MI, 10% with CHF #KidneyWk12
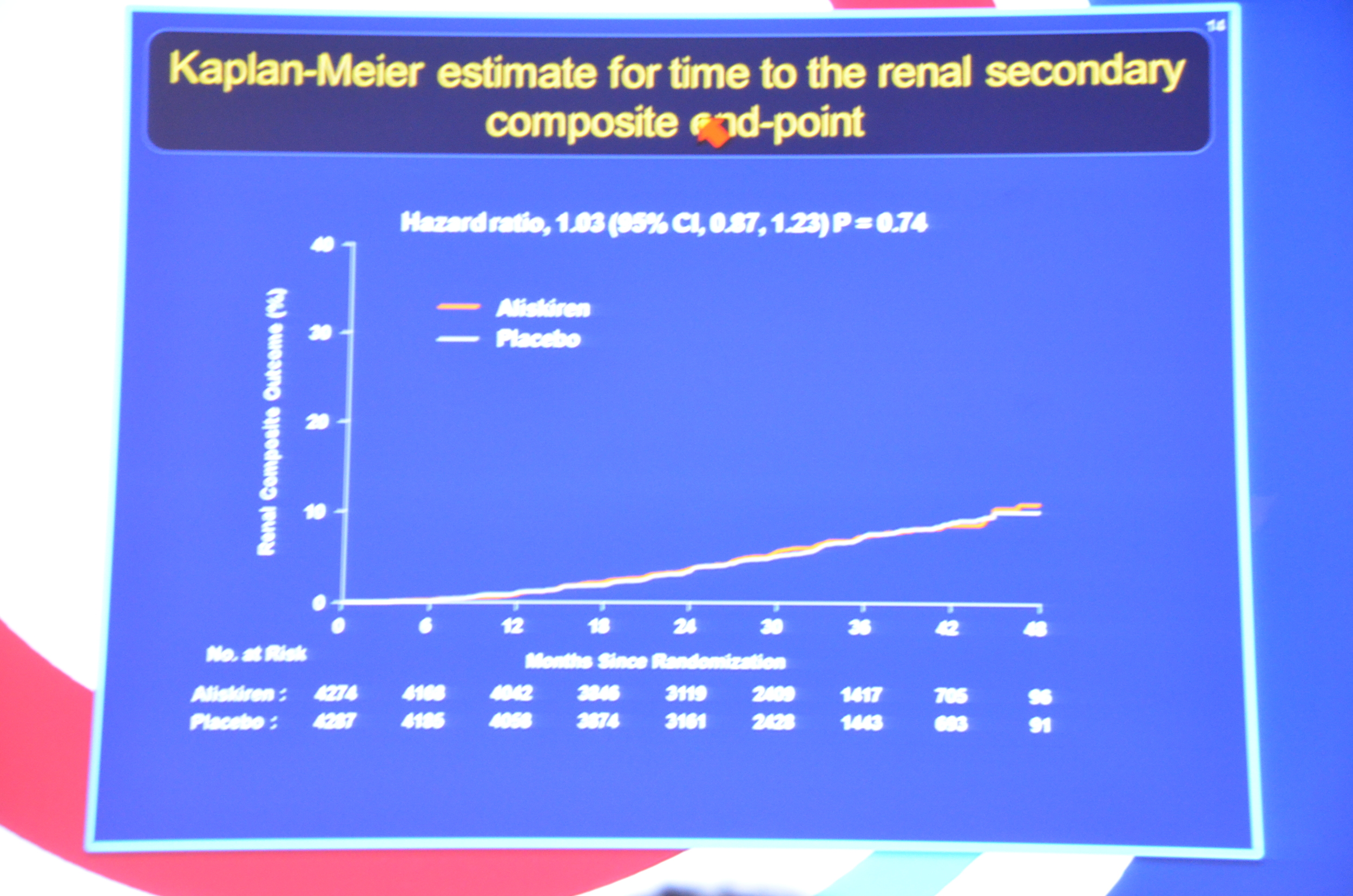
eAJKD No different between placebo and aliskiren for primary or secondary endpoint #KidneyWk12
eAJKD Mortality 1.06 HR with P=0.42, so no significance but trending toward bad things with aliskiren #KidneyWk12
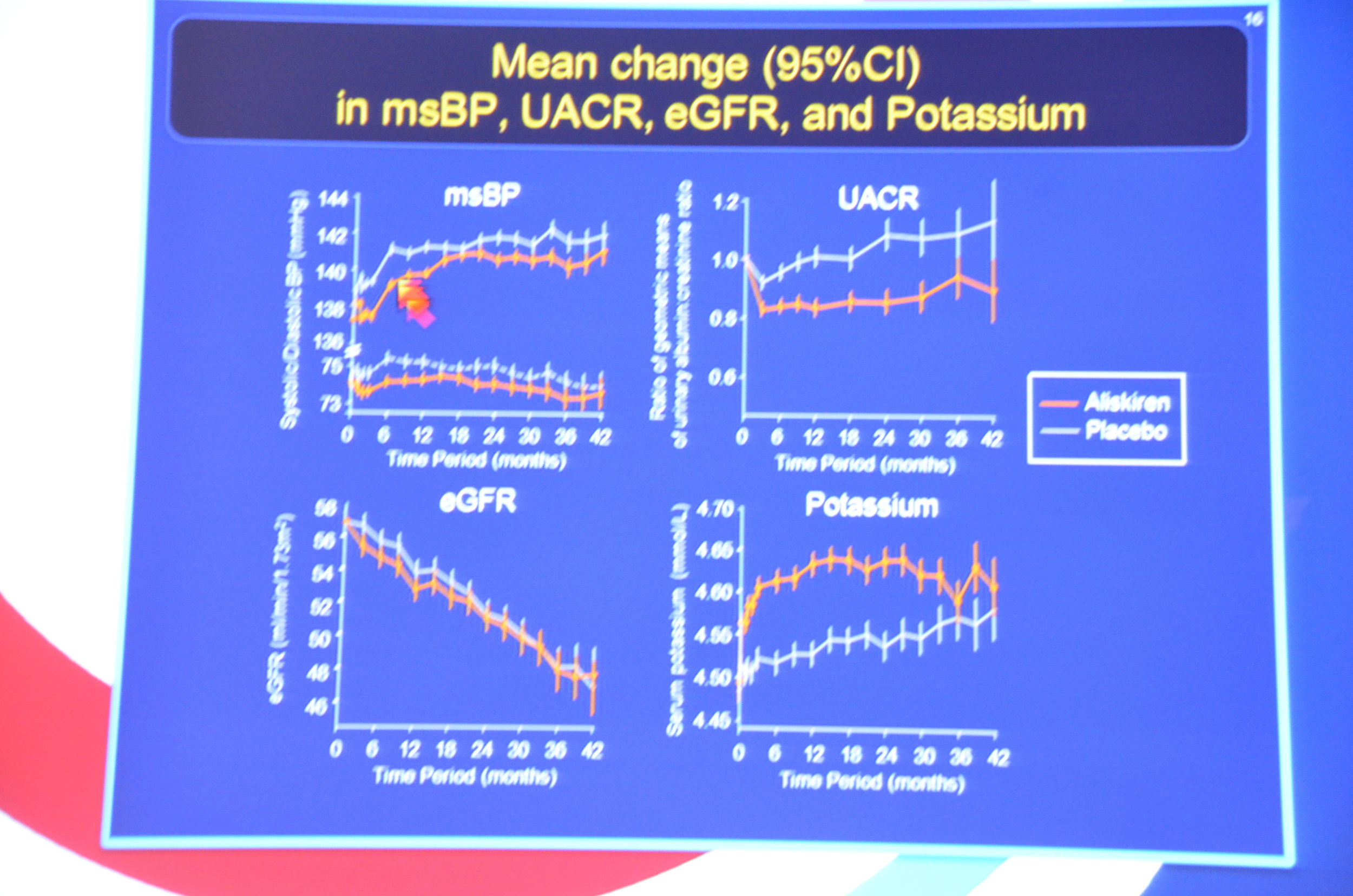
eAJKD Looks like aliskiren had a bit lower BP (1mmHg), lower albuminuria by 11% and increased K (0.15 mmol/L). No diff in eGFR. #KidneyWk12
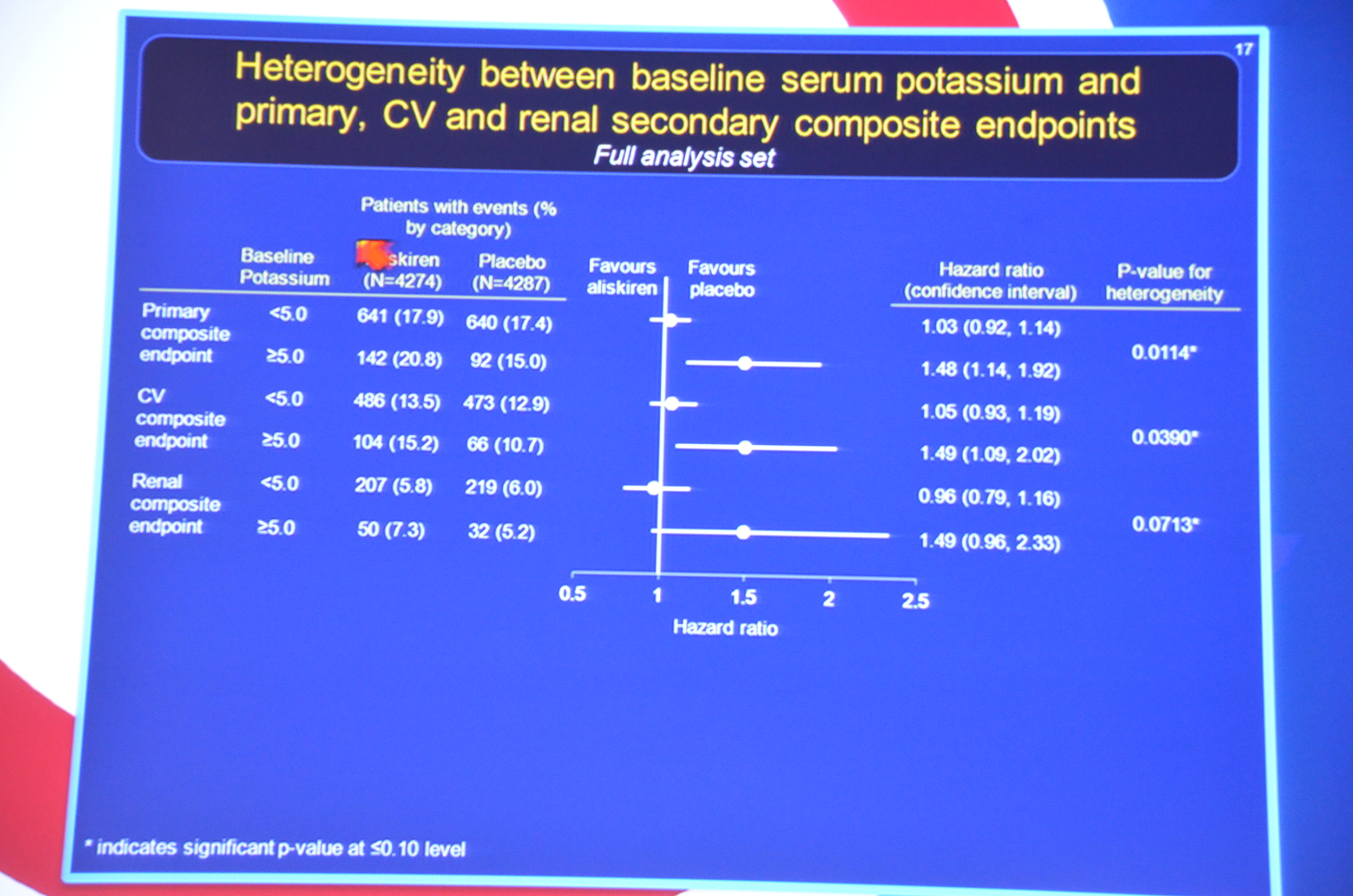
eAJKD Pts starting K >5 at baseline had worse CV and renal endpoint p=0.014. Protocol should have excluded them #KidneyWk12
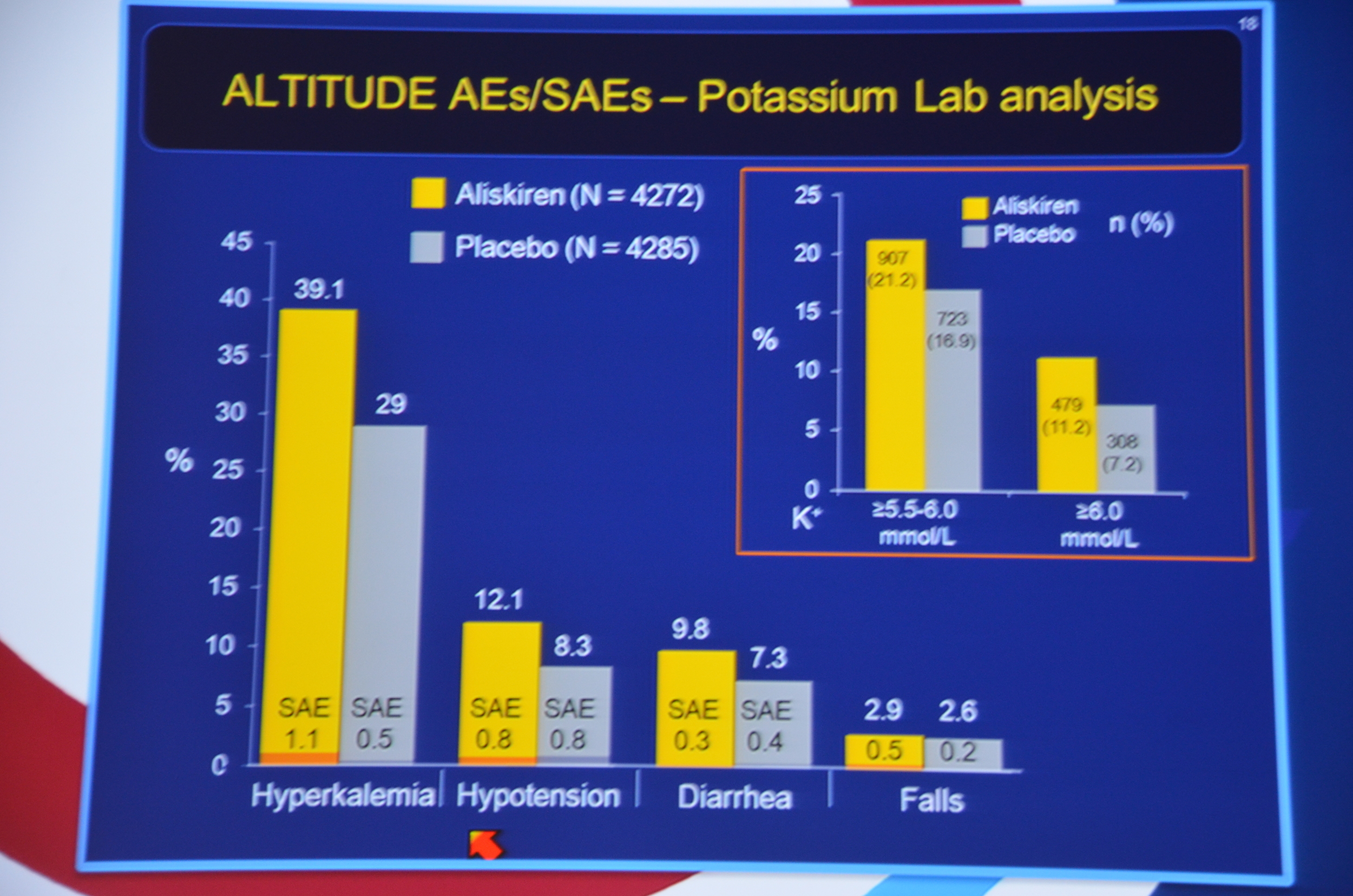
eAJKD 11% vs 7% Potassium > 6. #KidneyWk12
eAJKD Aliskiren may be harmful in type 2 DM. #KidneyWk12
eAJKD Study has dropped in the NEJM #KidneyWk12



